Abstract
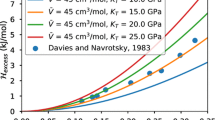
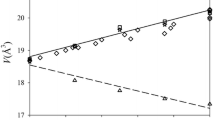
The volumes and enthalpies of mixing, ΔV Mix and ΔH Mix, of binary solid-solution aluminosilicate garnets have been studied by computer simulation. The use of “average atoms” to simulate solid solution was found to give results that are considerably different from those obtained by calculating and averaging over many configurations of cations at a given composition. Although we expect mineral properties calculated from model calculations to be correct only on a qualitative rather than a quantitative scale, fair agreement with experiment was obtained where carefully tested potential parameters were used. The results show that mixing behaviour in these materials is controlled by local strain and relaxation effects resulting from the atomic size mismatch of the mixing divalent cations. In particular, ΔV Mix and ΔH Mix are shown to scale quadratically with the volume difference between the end members, and to vary essentially symmetrically with composition, with a moderate dependence on the degree and nature of cation order. We conclude that computer modelling should be useful in providing detailed qualitative information about the mixing properties of solid solutions, which can help to better constrain and interpret experimental results.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 8 March 2000 / Accepted: 1 October 2000
Rights and permissions
About this article
Cite this article
Bosenick, A., Dove, M., Heine, V. et al. Scaling of thermodynamic mixing properties in garnet solid solutions. Phys Chem Min 28, 177–187 (2001). https://doi.org/10.1007/s002690000141
Issue Date:
DOI: https://doi.org/10.1007/s002690000141