Abstract
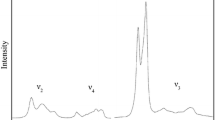
Infrared and Raman spectra of the ABX4 compound anglesite (PbSO4) were collected to 43 GPa at 300 K in three separate pressure media. A major transition in the spectra initiates at 23 GPa that is characterized by shifting and/or splitting of most of the fundamental vibrations of the sulfate group. On decompression, PbSO4 reverts to the ambient anglesite (barite) structure. This transition is kinetically impeded within more hydrostatic pressure media and does not proceed to completion within a neon medium even at pressures as high as 43 GPa. The previously observed spectral shift at 13 GPa occurs in less hydrostatic media (such as 4:1 methanol:ethanol), and involves subtle splitting of a few modes, particularly the (SO4)2− tetrahedral stretching and bending-derived Raman and infrared modes. Another possible shift occurs near 33 GPa, and primarily involves the appearance of new sulfate antisymmetric bending modes in the infrared spectrum and the onset of new sulfate antisymmetric stretching modes in the Raman spectrum. It is likely that PbSO4 begins to convert sluggishly to the P212121 ABX4 structure starting at 23 GPa, with a possible additional crystallographic distortion or shift in compression mechanism occurring near 33 GPa. The spectral shift at 13 GPa in non-hydrostatic media may be a differential stress-induced structural distortion, or a subtle transition that is not accessible within more hydrostatic media. Our results emphasize that apparently equilibrium transitions such as that at 23 GPa may be notably suppressed within more hydrostatic media.


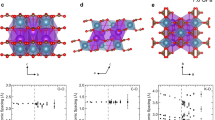
Modified from Errandonea and Manjon (2008), including additions from Santamaria-Perez et al. (2011)








Similar content being viewed by others
References
Alia JM, Edwards HGM, Lopez-Andres S, Gonzalez-Martin P, Garcia-Navarro FJ, Mansour HR (2000) FT-Raman study of three synthetic solid solutions formed by orthorhombic sulfates: celestite-barytes, barytes-anglesite and celestite-anglesite. Spectrosc Lett 33:323–336
Antao SM (2012) Structural trends for celestite (SrSO4), anglesite (PbSO4), and barite (BaSO4): confirmation of expected variations within the SO4 groups. Am Miner 97:661–665
Bastide JP (1987) Simplified systematics of the compounds ABX4 (X = O2–, F–) and possible evolution of their crystal structures under pressure. J Solid State Chem 71:115–120
Bradbury SE, Williams Q (2009) X-ray diffraction and infrared spectroscopy of monazite-structured CaSO4 at high pressures: implications for shocked anhydrite. J Phys Chem Solids 70:134–141
Buzgar N, Buzatu A, Sanislav IV (2009) The Raman study on certain sulfates. Geologie 55:5–23
Clavier N, Podor R, Dacheux N (2011) Crystal chemistry of the monazite structure. J Eur Ceram Soc 31:941–976
Crichton WA, Merlini M, Hanfland M, Muller H (2011) The crystal structure of barite, BaSO4, at high pressure. Am Miner 96:364–367
Dawson P, Hargreave MM, Wilkinson GR (1977) Polarized i.r. reflection, absorption and laser Raman studies on a single crystal of BaSO4. Spectrochim Acta 33A:83–93
Errandonea D, Majon FJ (2008) Pressure effects on the structural and electronic properties of ABX4 scintillating crystals. Prog Mater Sci 53:711–773
Errandonea D, Munoz A, Rodriguez-Hernandez P, Proctor JE, Sapina F, Bettinelli M (2015) Theoretical and experimental study of the crystal structures, lattice vibrations, and band structures of monazite-type PbCrO4, PbSeO4, SrCrO4, SrSeO4. Inorg Chem 54:7524–7535
Evans KA (2012) The redox budget of subduction zones. Earth Sci Rev 113:11–32
Evans KA, Tomkins AG, Cliff J, Fiorentini ML (2014) Insights into subduction zone sulfur recycling from isotopic analysis of eclogite-hosted sulfides. Chem Geol 365:1–19
Fujii T, Ohfuji H, Inoue T (2016) Phase relation of CaSO4 at high pressure and temperature up to 90 GPa and 2300 K. Phys Chem Miner 43:353–361
Fukunaga O, Yamaoka S (1979) Phase transformations in ABO4 type compounds under high pressure. Phys Chem Miner 5:167–177
Gracia L, Beltran A, Errandonea D, Andres J (2012) CaSO4 and its pressure-induced phase transitions. A density function theory study. Inorg Chem 51:1751–1759
Griffith WP (1970) Raman studies on rock-forming minerals. Part II. Minerals containing MO3, MO4 and MO6 groups. J Chem Soc A:286–291
Hazen RM, Finger LW, Mariathasan JWE (1985) High-pressure crystal chemistry of scheelite-type tungstates and molybdates. J Phys Chem Solids 46:253–263
Igartua JM, Aroyo MI, Perez-Mato JM (1996) Systematic search of materials with high-temperature structural phase transitions: application to space group P2 1 2 1 2 1. Phys Rev B 54:12744–12752
Jacobsen SD, Smyth RD, Swope RJ, Downs RD (1998) Rigid-body character of the SO4 groups in celestine, anglesite and barite. Can Miner 36:1053–1060
Jayasooriya UA, Kettle SFA, Mahasuverachai S, Al-Jowder O (1991) Manifestation of latent space group symmetry in the vibrational spectra of MSO4; where M = Ba, Sr, and Pb. J Chem Phys 94:5946–5948
Jayasooriya UA, Kettle SFA, Mahasuverachai S, White RP (1996) Lattice vibrations in compounds crystallizing with baryte structure. J Chem Soc Faraday Trans 92:3625–3628
Kaminskii AA, Bohaty L, Becker P, Rhee H, Eichler HJ, Lux O, Koltashev VV (2011) Stimulated Raman scattering in natural crystals on SrSO4, BaSO4 and PbSO4: high-order Stokes and anti-stokes generation with single-crystal wavelength UV, visible, and near-IR excitation, as well as cascaded up-conversion nonlinear x(3) – x(3) lasing effects under dual-wavelength picosecond collinear coherent pumping. Appl Phys 105:363–378
Klotz S, Chervin JC, Munsch P, Le Marchand G (2009) Hydrostatic limits of 11 pressure transmitting media. J Phys D Appl Phys 42:075413
Knittle E, Williams Q (1993) High pressure Raman spectroscopy of ZrSiO4: observation of the zircon to scheelite transition at 300 K. Am Miner 78:245–252
Krasnyanskii GE, Tsyashchenko YP (1980) Contribution of different intermolecular potentials to the Davydov splitting of vibrational levels in crystals and site symmetry. Phys Status Solidi B 101:K151–K153
Lane MD (2007) Mid-infrared emission spectroscopy of sulfate and sulfate bearing minerals. Am Miner 92:1–18
Lee P-L, Huang E, Yu S-C, Chen Y-H (2013) High-pressure Raman study on anglesite. World J Condens Matter Phys 3:28–32
Li B, Xu J, Chen W, Ye Z, Huang S, Fan D, Zhou W, Xie H (2018) Compressibility and expansivity of anglesite (PbSO4) using in situ synchrotron X-ray diffraction at high-pressure and high-temperature conditions. Phys Chem Miner. https://doi.org/10.1007/s00269-018-0970-1
Lopez-Solano J, Rodriguez-Hernandez P, Munoz A, Gomis O, Santamaria-Perez D, Errandonea D, Manjon FJ, Kumar RS, Stavrou E, Raptis C (2010) Theoretical and experimental study of the structural stability of TbPO4 at high pressures. Phys Rev B 81:144126
Mao HK, Xu J, Bell PM (1986) Calibration of the ruby pressure gauge to 800 kbar under quasi-hydrostatic conditions. J Geophys Res 91:4673–4676
Miyake M, Minato I, Morikawa H, Iwai S-I (1978) Crystal structures and sulphate force constants of barite, celestite, and anglesite. Am Miner 63:506–510
Ni Y, Hughes JM, Mariano AN (1995) Crystal chemistry of the monazite and xenotime structures. Am Miner 80:21–26
Porto SPS, Scott JF (1967) Raman spectra of CaWO4, SrWO4, and SrMoO4. Phys Rev 157:716–718
Santamaría-Pérez D, Chuliá-Jordán R (2012) Compression of mineral barite, BaSO4: a structural study. High Press Res 32:81–88
Santamaría-Pérez D, Gracia L, Garbarino G, Beltran A, Chuliá-Jordán R, Gomis O, Errandonea D, Ferrer-Roca C, Martinez-Garcia D, Segura A (2011) High-pressure study of the behavior of mineral barite by X-ray diffraction. Phys Rev (B) 84:054102
Silva EN, Ayala AP, Guedes I, Paschoal CWA, Moreira RL, Loong C-K, Boatner LA (2006) Vibrational spectra of monazite-type rare earth orthophosphates. Opt Mater 29:224–230
Vandenborre MT, Michel D, Ennaciri A (1989) Vibrational spectra and force fields of scheelite-type germanates. Spectrochim Acta Part A Mol Spectrosc 45:721–727
Acknowledgements
We thank Dan Sampson for help with the infrared and Raman spectrometers, and two reviewers for helpful comments that markedly improved the paper. We thank Andrew Doran for assistance loading neon. Work partially supported by NSF through EAR-1620423 and EAR-1522560 and COMPRES, the Consortium for Materials Properties Research in Earth Sciences under NSF Cooperative Agreement EAR-1606856. Part of this work was performed under the auspices of the US Department of Energy by Lawrence Livermore National Laboratory under Contract No. DE-AC52-07NA27344.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sawchuk, K., O’Bannon, E.F., Vennari, C. et al. An infrared and Raman spectroscopic study of PbSO4-anglesite at high pressures. Phys Chem Minerals 46, 623–637 (2019). https://doi.org/10.1007/s00269-019-01027-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-019-01027-z