Abstract
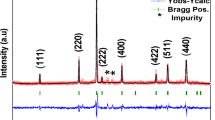
An extensive characterisation of the magnetic properties of synthetic powders of kuramite, with formal composition Cu3SnS4, was performed. Powders were investigated through superconducting quantum interference device (SQUID) magnetometry, electron paramagnetic resonance (EPR) spectroscopy, X-ray powder diffraction (XRPD), scanning and transmission electron microscopies (SEM and TEM) and microanalysis. SEM and TEM reveal the presence of nanodimensioned particles. XRPD clearly shows that Cu3SnS4 crystallised in a cubic sphalerite-type structural model, in spite of the stannite-type tetragonal structure described for the natural phase. This difference arises from a full random distribution of cations. Synthetic kuramite nanopowders exhibit a marked paramagnetism, originated by the presence of Cu(II), definitely assessed by EPR measurements. Moreover, the overall magnetic behaviour of the sample cannot be simply ascribed to diluted paramagnetism, and this suggests the presence of strong superexchange interactions among Cu(II) ions even at room temperature. The main consequences of these results are the definitive assessment of the chemical formula Cu(I)2Cu(II)SnS4 and of a random distribution of Cu(II), Cu(I) and Sn(IV) ions within the available tetrahedral sites.






Similar content being viewed by others
References
Abragam A, Bleaney B (1970) Electron paramagnetic resonance of transition ions. Clarendon Press, Oxford, pp 527–529
Bernardini GP, Borrini D, Caneschi A, Di Benedetto F, Gatteschi D, Ristori S, Romanelli M (2000) EPR and SQUID magnetometry study of Cu2FeSnS4 (stannite) and Cu2ZnSnS4 (kesterite). Phys Chem Miner 27:453–461
Bouaziz M, Ouerfelli J, Amlouk M, Belgacem S (2007) Structural and optical properties of Cu3SnS4 sprayed thin films. Phys Stat Sol (a) 204(10):3354–3360
Caneschi A, Cipriani C, Di Benedetto F, Sessoli R (2004) Characterisation of the antiferromagnetic transition of Cu2FeSnS4, the synthetic analogue of stannite. Phys Chem Miner 31:190–193
Chen XY, Wang X, An CH, Liu JW, Qian YT (2003) Preparation and characterization of ternary Cu–Sn–E (E = S, Se) semiconductor nanocrystallites via a solvothermal element reaction route. J Cryst Growth 256:368–376
Di Benedetto F, Evstigneeva T, Borgheresi M, Caneschi A, Romanelli M (2009) The unusual magnetic properties of kuramite–stannite pseudobinary series: a SQUID and EPR survey. Phys Chem Minerals 36:301–309
Evstigneeva TL, Rusakov VS, YuK Kabalov (2003) Isomorphism in the minerals of stannite-family. New Data Mineral 38:65–69
Fernandes PA, Salomé PMP, da Cunha AF (2010) A study of ternary Cu2SnS3 and Cu3SnS4 thin films prepared by sulfurizing stacked metal precursors. J Phys D Appl Phys 43:215403 (11 pp)
Fleisher M, Cabri LJ, Chao GY, Pabst A (1980) New mineral names. Am Mineral 65:1065–1069
Fries T, Shapira Y, Palacio F, Moron MC, McIntyre GJ, Kershaw R, Wold A, McNiff EJ Jr (1997) Magnetic ordering of the antiferromagnet Cu2MnSnS4 from magnetisation and neutron scattering measurements. Phys Rev (B) 56(9):5424–5431
Hergert F, Hock R (2007) Predicted formation reactions for the solid-state syntheses of the semiconductor materials Cu2SnX3 and Cu2ZnSnX4 (X = S, Se) starting from binary chalcogenides. Thin Solid Films 515:5953–5956
Hu H, Liu Z, Yang B, Chen X, Qian Y (2005) Template-mediated growth of Cu3SnS4 nanoshell tubes. J Cryst Growth 284:226–234
Hu HM, Deng CH, Sun M, Zhang KH (2010) Solvothermal synthesis of hierarchical structured chinese rose-shaped Cu3SnS4 microspheres. Chinese J Inorg Chem 26(7):1189–1194 (English abstract)
Kovalenker VA (1981) Kuramite, Cu3SnS4, a new mineral of the stannite group. Int Geol Rev 23(3):365–370
Kovalenker VA, Evstigneeva TL, Troneva NV, Vyal’sov LN (1979) Kuramite, Cu3SnS4, a new mineral of the stannite group. Zap Vses Miner Obsh 108:564–569 (in Russian)
Kulikova IM, Evstigneeva TL, Bortnikov NS (2005) Study of chemical bonding of copper atoms in minerals of the stannite group, kuramite–stannite series. Dokl Earth Sci 401(3):403–405
Moh GM (1960) Experimentelle Untersuchungen am Zinnkiesen und analogen Germaniumverbindungen. N Jb Mineral Abh 94:1125–1144
Peisach J, Blumberg WD (1974) Analysis of EPR Copper: structural implications derived from the analysis of EPR spectra of natural and artificial Cu-proteins. Arch Biochem Biophys 165:691–708
Rodriguez-Carvajal J (1993) Recent advances in magnetic structure determination by neutron powder diffraction. Physica B. 192:55–69
Rusakov VS, Chistyakova NI, Burkovsky IA, Gapochka AM, Evstigneeva TL (2010) Mössbauer study of compounds of Cu3–xFexSnS4 and Cu2Fe1–xZnxSnS4 systems. Bull Russian Acad Sci Phys 74(3):389–393
Sykes MF, Gaunt DS, Glen M (1976) Percolation processes in three dimension. J Phys A Math Gen 9(10):1705–1712
Xiong YJ, Xie Y, Du GA, Su HL (2002) From 2D framework to quasi-1D nanomaterial: preparation, characterization, and formation mechanism of Cu3SnS4 nanorods. Inorg Chem 41:2953–2959
Zalewski W, Bacewicz R, Antonowicz J, Pietnoczka A, Evstigneeva TL, Schorr S (2010) XAFS study of kesterite, kuramite and stannite type alloys. J Alloys Compd 492:35–38
Acknowledgments
The authors want to express their warmest thanks to S. Bellandi, F. Capolupo and A. De Luca (Univ. Florence) for their assistance during the syntheses, to M. Paolieri and M. Ulivi (Univ. Florence) for their help in centrifugation and SEM investigations, and to L. Sorace (Univ. Florence) for the EPR measurements. Authors are also indebted to G.P. Bernardini for the stimulating discussion and enthusiastic support to this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
This manuscript is dedicated to Gian Piero Bernardini in occasion of his 80th birthday.
Rights and permissions
About this article
Cite this article
Benedetto, F.D., Borrini, D., Caneschi, A. et al. Magnetic properties and cation ordering of nanopowders of the synthetic analogue of kuramite, Cu3SnS4 . Phys Chem Minerals 38, 483–490 (2011). https://doi.org/10.1007/s00269-011-0421-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-011-0421-8