Abstract
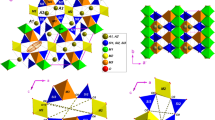
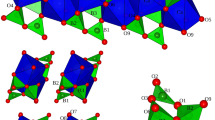
The stability and the thermo-elastic behaviour of a natural londonite
has been investigated up to 1,273(3) K (at 0.0001 GPa) and up to 4.85(5) GPa (at 298 K) by means of in situ X-ray powder diffraction. Up to 973 K, no evidence of phase transition or anomalous thermo-elastic behaviour was observed. At T > 973 K, londonite shows the first evidence of an irreversible structure destabilisation. The volume thermal expansion coefficient between 298 and 973 K is α0 = 2.38(6)·10−5 K−1. Londonite shows an elastic behaviour up to 4.85 GPa. No phase transition has been observed within the pressure range investigated. P–V data fitted with a second-order Birch–Murnaghan equation of state give V 0 = 389.1(1)Å3 and K T0 = 280(12) GPa. On the basis of the good thermo-elastic behaviour, substantiated by the significantly low compressibility, the modest thermal expansion up to 1,000 K and the significantly high amount of boron (B2O3 ~ 50wt%), londonite-type materials could be considered as potential inorganic host for 10B in composite neutron-absorbing materials.




Similar content being viewed by others
References
Angel RJ (2000) Equation of state. In: Hazen RM, Downs RT (eds) High-temperature and high-pressure crystal chemistry. Reviews in mineralogy and geochemistry, vol 41. Mineralogical Society of America and Geochemical Society, Washington, pp 35–59
Angel RJ (2001) EOS-FIT V6.0. Computer program. Crystallography Laboratory, Dept. Geological Sciences. Virginia Tech, Blacksburg
Angel RJ, Bujak M, Zhao J, Gatta GD, Jacobsen SD (2007) Effective hydrostatic limits of pressure media for high-pressure crystallographic studies. J Appl Crystallogr 40:26–32
Barth RF, Solloway AH, Fairchild RG (1990) Boron neutron capture therapy of cancer. Cancer Res 50:1061–1070
Birch F (1947) Finite elastic strain of cubic crystal. Phys Rev 71:809–824
Carter RS, Palevsky H, Myers VW, Hughes DJ (1953) Thermal neutron absorption cross sections of boron and gold. Phys Rev 92:716–721
Chervin JC, Canny B, Mancinelli M (2001) Ruby-spheres as pressure gauge for optically transparent high pressure cells. High Press Res 21:305–314
Donnay G, Thorpe AN, Senftle FE, Sioda R (1966) Absence of neutral alkali atoms in rhodizite. Science 154:889–890
Donnay G, Thorpe AN, Sioda R, Senftle FE (1967) The formula of rhodizite. Carnegie Inst Washington Yearbook 65:299–300
Frondel C, Ito J (1965) Composition of rhodizite. Tschermaks Mineralogische und Petrographische Mitteilungen 10:409–412
Fujii T, Mori Y, Hyodo H, Kimura K (2010) X-ray diffraction study of B4C under high pressure. J Phys Conf Ser 215: 012011 (doi:10.1088/1742-6596/215/1/012011)
Gatta GD, Rotiroti N, Fisch M, Kadiyski M, Armbruster T (2008a) Stability at high-pressure, elastic behaviour and pressure-induced structural evolution of CsAlSi5O12, a potential nuclear waste disposal phase. Phys Chem Miner 35:521–533
Gatta GD, Rotiroti N, Zanazzi PF, Rieder M, Drabek M, Weiss Z, Klaska R (2008b) Synthesis and crystal structure of the feldspathoid CsAlSiO4: an open-framework silicate and potential nuclear waste disposal phase. Am Miner 93:988–995
Gatta GD, Rinaldi R, McIntyre GJ, Nénert G, Bellatreccia F, Guastoni A, Della Ventura G (2009) On the crystal structure and crystal chemistry of pollucite, (Cs, Na)16Al16Si32O96·nH2O: a natural microporous material of interest in nuclear technology. Am Miner 94:1560–1568
Gatta GD, Vignola P, McIntyre GJ, Diella V (2010a) On the crystal chemistry of londonite [(Cs, K, Rb)Al4Be5B11O28]: a single-crystal neutron diffraction study at 300 and 20 K. Am Miner 95:1467–1472
Gatta GD, Rotiroti N, Fisch M, Armbruster T (2010b) Stability at high pressure, elastic behavior and pressure-induced structural evolution of ‘‘Al5BO9’’, a mullite-type ceramic material. Phys Chem Minerals 37:227–236
Gualtieri A (1996) Modal analysis of pyroclastic rocks by combined Rietveld and RIR methods. Powder Diffr 11:1–10
Hammersley AP, Svensson SO, Hanfland M, Fitch AN, Häusermann D (1996) Two-dimensional detector software: from real detector to idealized image or two-theta scan. High Press Res 14:235–245
Heinz DL, Jeanloz R (1984) The equation of state of the gold calibration standard. J Appl Phys 55:885–893
Larson AC, Von Dreele RB (1994) General structure analysis system (GSAS), Los Alamos Natl Lab Rept LAUR 86–748
Laurs BM, Pezzotta F, Simmons WB, Falster AU, Muhlmeister S (2002) Rhodizite-londonite from the Antsongombato pegmatite, Central Madagascar. Gems Gemol 38:326–339
Lazzari R, Vast N, Besson JM, Baroni S, Dal Corso A (1999) Atomic structure and vibrational properties of icosahedral B4C boron carbide. Phys Rev Lett 83:3230–3233
Le Bail A, Duroy H, Fourquet JL (1988) Ab initio structure determination of LiSbWO6 by X-ray powder diffraction. Mat Res Bull 23:447–452
Machiels A, Lambert R (2005) Handbook on neutron absorber materials for spent nuclear fuel applications: 2005 Edition—1011818. Electric Power Research Institute. Palo Alto, California
Mao HK, Xu J, Bell PM (1986) Calibration of the ruby pressure gauge to 800 kbar under quasi-hydrostatic conditions. J Geophys Res 91:4673–4676
Miletich R, Allan DR, Kush WF (2000) High-pressure single-crystal techniques. In: Hazen RM, Downs RT (eds) High-temperature and high-pressure crystal chemistry, reviews in mineralogy and geochemistry, vol 41. Mineralogical Society of America and Geochemical Society, Washington, pp 445–519
Pezzotta F (2001) Madagascar—a mineral and gemstone paradise. Extra Lapis Engl 1:97
Pezzotta F (2005) First attempt to the petrogenesis and classification of granitic pegmatites of the Itremo Region (Madagascar). In: Proceedings of the international meeting on crystallization processes in granitic pegmatites, Elba Island (Ialy), May 23–28 [http://www.minsocam.org/MSA/Special/Pig/PIG_articles/PIG_articles.html]
Pring A, Din VK, Jefferson DA, Thomas JM (1986) The crystal chemistry of rhodizite: a re-examination. Min Mag 50:163–172
Rauch H, Waschkowski W (2002) Neutron scattering lengths. In: Dianoux AJ, Lander G (eds) Neutron data booklet, 1st edn. Institute Laue-Langevin, Grenoble (F), pp 1–18
Rietveld HM (1969) A profile refinement method for nuclear and magnetic structures. J Appl Crystallogr 2:65–71
Scholze H (1956) Über aluminiumborate. Z Anorg Allg Chem 284:272–277
Simmons WB, Pezzotta F, Falster AU, Webber KL (2001) Londonite, a new mineral species: the Cs-dominant analogue of rhodizite from the Antandrokomby granitic pegmatite, Madagascar. Can Mineral 39:747–755
Taxer KJ, Buerger MJ (1967) The crystal struture of rhodizite. Z Kristallogr 125:423–436
Thomson P, Cox DE, Hastings JB (1987) Rietveld refinement of Debye-Scherrer synchrotron X-ray data from Al2O3. J Appl Crystallogr 20:79–83
Acknowledgments
The authors are grateful to N. Marinoni and E. Ferrari (Milan). The research was financially supported by University of Milan (PUR2009) and CNR-IDPA. Y. Lee thanks the support by the National Research Foundation through the Nuclear R&D Program (Grant No. M2AM06-2008-03931). Experiments at PAL were supported by Ministry of Education, Science and Technology of the Korean Government and POSTECH. Two anonymous reviewers and the Editor M. Rieder are thanked for their suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gatta, G.D., Vignola, P. & Lee, Y. Stability of (Cs, K)Al4Be5B11O28 (londonite) at high pressure and high temperature: a potential neutron absorber material. Phys Chem Minerals 38, 429–434 (2011). https://doi.org/10.1007/s00269-011-0416-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-011-0416-5