Abstract
Introduction
Augmentation mammoplasty is one of the most popular aesthetic operations in the world. In Korea, one of the fillers used for breast augmentation is AQUAfilling® gel (Biomedica. spol, s,r,o, Czech Republic). AQUAfilling® gel is a hydrophilic gel composed of 98% sodium chloride solution (0.9%) and 2% cation copolyamide.
Methods
This is a case report describing a patient that suffered complications after AQUAfilling® gel injection for breast augmentation.
Results
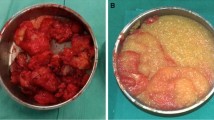
A 32-year-old female patient was referred to our plastic surgery department with a chief complaint of repeated wound dehiscence and fluid discharge involving both breasts. She was treated via surgical intervention for removal of necrotic infected tissue and filler, as well as massive irrigation three times. After the third surgery, there were no complications, including infection or dehiscence, during a 1-year follow-up period.
Conclusions
Although AQUAfilling® gel is easy to inject and is natural looking, once a complication occurs, treatment is difficult. Also, there are concerns regarding toxicity of the gel material and its influence on surrounding tissues. Hence, sufficient evidences of long-term safety must be accumulated and proved, until which time the aesthetic use of the unapproved filler must be restricted.
Level of Evidence V
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.





Similar content being viewed by others
References
Shin JH, Suh JS, Yang SG (2015) Correcting shape and size using temporary filler after breast augmentation with silicone implants. Arch Aesthetic Plast Surg 21:124–126
Amin Snehal P, Marmur Ellen S, Goldberg David J (2004) Complications from injectable polyacrylamide gel, a new nonbiodegradable soft tissue filler. Dermatol Surg 30(12 Part 2):1507–1509
Christensen Lise H, Breiting V, Aasted A, Jørgensen J, Kebuladze I (2003) Long term effects of polyacrylamide hydrogel (PAAG, Interfall/Contura SA) in human breast tissue. Plast Reconstr Surg 111:1883–1890
US Food and Drug Administration (2015) Soft tissue fillers: dermal fillers. US Food and Drug Administration, Silver Spring
Roh TS (2016) Position statement of Korean Academic Society of Aesthetic and Reconstructive Breast Surgery: concerning the use of Aquafilling® for breast augmentation. Arch Aesthetic Plast Surg 22:45–46
Arslan Gozde, Celik Levent, Atasoy Mehmet Mahir, Celik Levent, Cubuk Rahmi (2017) Complication of non-US guided procedure of aquafilling breast gel. Med Ultrason 19(2):236–240
Okubo M, Hyakusoku H, Kanno K, Fumiiri M (1992) Complications after injection mammaplasty. Aesthetic Plast Surg 16:181–187
Ohtake N, Koganei Y, Itoh M, Shioya N (1989) Postoperative sequel of augmentation mammaplasty by injection method in Japan. Aesth Plast Surg 13:67
Breiting V, Aasted A, Jørgensen A (2004) A study on patients treated with polyacrylamide hydrogel injection for facial corrections. Aesthetic Plast Surg 28:45–53
Peters W, Fornasier V (2009) Complications from injectable materials used for breast augmentation. Can J Plast Surg 17:89–96
Patlazhan G, Unukovych D, Pshenisnov K (2013) Breast reconstruction and treatment algorithm for patients with complications after polyacrylamide gel injections: a 10-year experience. Aesthetic Plast Surg 37:312–320
Ono S, Ogawa R, Hyakusoku H (2010) Complications after polyacrylamide hydrogel injection for soft-tissue augmentation. Plast Reconstr Surg 126:1349–1357
Smith EA, Oehme FW (1991) Acrylamide and polyacrylamide: a review of production, use, environmental fate and neurotoxicity. Rev Environ Health 9:215–228
Dearfield KL, Douglas GR, Ehling UH (1995) Acrylamide: a review of its genotoxicity and an assessment of heritage genetic risk. Mutat Res 330:71–99
Lin WC, Hsu GC, Hsu YC (2008) A late complication of augmentation mammoplasty by polyacrylamide hydrogel injection: ultrasound and magnetic resonance imaging findings of huge galactocele formation in a puerperal woman with pathological correlation. Breast J 14:584–587
Kneeshaw PJ, Lowry M, Manton D (2006) Differentiation of benign from malignant breast disease associated with screening detected microcalcifications using dynamic contrast enhanced magnetic resonance imaging. Breast 15:29–38
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Rights and permissions
About this article
Cite this article
Jung, B.K., Yun, I.S., Kim, Y.S. et al. Complication of AQUAfilling® Gel Injection for Breast Augmentation: Case Report of One Case and Review of Literature. Aesth Plast Surg 42, 1252–1256 (2018). https://doi.org/10.1007/s00266-018-1107-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-018-1107-0