Abstract
Background
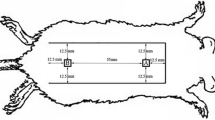
No effective treatments have been found for flap necrosis. Animal models that focus on the initial flap viability are inappropriate for necrotic wound studies. Keratinocyte growth factor (KGF) promotes keratinocyte proliferation with stronger activity and fewer complications and thus may be useful for necrotic flap wound healing.
Methods
Rats with modified flap necrosis were randomly divided into four groups. An adenoviral vector expressing KGF was injected subdermally in the back of the animals after necrosis began. The expression and effect of KGF was assessed by real-time polymerase chain reaction, enzyme-linked immunoassay, and transwell, and wound healing was monitored.
Results
The plasmid and adenovirus were able to express KGF and stimulate epithelial cell growth (p = 0.029). Histology showed that the necrosis healed fastest in the KGF administration group than in the control groups (p < 0.01). The adenovirus-mediated KGF (Ad-KGF) group had the thickest epithelium on days 15 (p = 0.044) and 25 (p = 0.014). The KGF level in the blood serum soared 10 and 15 days postoperatively (p < 0.01) but returned to baseline by day 25 (p = 0.561). The KGF mRNA levels in vivo increased dramatically in the Ad-KGF group (p = 0.037).
Conclusions
The extended flap model is applicable in necrotic wound study. Keratinocyte growth factor can promote secondary necrotic flap wound healing, and administration of KGF can be achieved by an adenoviral vector.
Level of Evidence IV
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.







Similar content being viewed by others
References
Lineaweaver WC, Lei MP, Mustain W, Oswald TM, Cui D, Zhang F (2004) Vascular endothelium growth factor, surgical delay, and skin flap survival. Ann Surg 239(866–873):873–875
Roman S, Poole M, Lindeman R (2004) Vascular endothelial growth factor (VEGF) expression and the effect of exogenous VEGF on survival of a random flap in the rat. Br J Plast Surg 57:174
Hallock GG (2001) Physiological studies using laser Doppler flowmetry to compare blood flow to the zones of the free TRAM flap. Ann Plast Surg 47:229–233
Akamatsu J, Ueda K, Tajima S, Nozawa M (2000) Sulfatide elongates dorsal skin flap survival in rats. J Surg Res 92:36–39
Karacal N, Ambarcioglu O, Topal U, Mamedov T, Kutlu N (2005) Enhancement of dorsal random-pattern skin flap survival in rats with topical lidocaine and prilocaine (EMLA): enhancement of flap survival by EMLA. J Surg Res 124:134–138
Handschin AE, Busch K, Vogt PM (2009) The efficacy of prophylactic low-molecular-weight heparin to prevent pulmonary thromboembolism in immediate breast reconstruction using the TRAM flap. Plast Reconstr Surg 124:322–323
Gurunluoglu R, Meirer R, Shafighi M, Huemer GM, Yilmaz B, Piza-Katzer H (2005) Gene therapy with adenovirus-mediated VEGF enhances skin flap prefabrication. Microsurgery 25:433–441
Haws MJ, Erdman D, Bayati S, Brown RE, Russell RC (2001) Basic fibroblast growth factor–induced angiogenesis and prefabricated flap survival. J Reconstr Microsurg 17(39–42):43–44
Kryger Z, Zhang F, Dogan T, Cheng C, Lineaweaver WC, Buncke HJ (2000) The effects of VEGF on survival of a random flap in the rat: Examination of various routes of administration. Br J Plast Surg 53:234–239
Rashid MA, Akita S, Razzaque MS, Yoshimoto H, Ishihara H, Fujii T, Tanaka K, Taguchi T (1999) Coadministration of basic fibroblast growth factor and sucrose octasulfate (sucralfate) facilitates the rat dorsal flap survival and viability. Plast Reconstr Surg 103:941–948
Taub PJ, Marmur JD, Zhang WX, Senderoff D, Nhat PD, Phelps R, Urken ML, Silver L, Weinberg H (1998) Locally administered vascular endothelial growth factor cDNA increases survival of ischemic experimental skin flaps. Plast Reconstr Surg 102:2033–2039
Ware LB, Matthay MA (2002) Keratinocyte and hepatocyte growth factors in the lung: roles in lung development, inflammation, and repair. Am J Physiol Lung Cell Mol Physiol 282:L924–L940
Takeoka M, Ward WF, Pollack H, Kamp DW, Panos RJ (1997) KGF facilitates repair of radiation-induced DNA damage in alveolar epithelial cells. Am J Physiol 272:L1174–L1180
Weigelt C, Haas R, Kobbe G (2011) Pharmacokinetic evaluation of palifermin for mucosal protection from chemotherapy and radiation. Expert Opin Drug Metab Toxicol 7:505–515
Finch PW, Rubin JS (2004) Keratinocyte growth factor/fibroblast growth factor 7, a homeostatic factor with therapeutic potential for epithelial protection and repair. Adv Cancer Res 91:69–136
Rubin JS, Osada H, Finch PW, Taylor WG, Rudikoff S, Aaronson SA (1989) Purification and characterization of a newly identified growth factor specific for epithelial cells. Proc Natl Acad Sci U S A 86:802–806
Werner S, Smola H, Liao X, Longaker MT, Krieg T, Hofschneider PH, Williams LT (1994) The function of KGF in morphogenesis of epithelium and reepithelialization of wounds. Science 266:819–822
Lawrence WT, Diegelmann RF (1994) Growth factors in wound healing. Clin Dermatol 12:157–169
Choi HI, Choi GI, Kim EK, Choi YJ, Sohn KC, Lee Y, Kim CD, Yoon TJ, Sohn HJ, Han SH et al (2011) Hair greying is associated with active hair growth. Br J Dermatol 165:1183–1189
Akilov OE, Donovan MJ, Stepinac T, Carter CR, Whitcomb JP, Hasan T, McDowell MA (2007) T helper type 1 cytokines and keratinocyte growth factor play a critical role in pseudoepitheliomatous hyperplasia initiation during cutaneous leishmaniasis. Arch Dermatol Res 299:315–325
Farrell CL, Rex KL, Chen JN, Bready JV, DiPalma CR, Kaufman SA, Rattan A, Scully S, Lacey DL (2002) The effects of keratinocyte growth factor in preclinical models of mucositis. Cell Prolif 35(Suppl 1):78–85
Hille A, Gruger S, Christiansen H, Wolff HA, Volkmer B, Lehmann J, Dorr W, Rave-Frank M (2010) Effect of tumour-cell-derived or recombinant keratinocyte growth factor (KGF) on proliferation and radioresponse of human epithelial tumour cells (HNSCC) and normal keratinocytes in vitro. Radiat Environ Biophys 49:261–270
Peng C, He Q, Luo C (2011) Lack of keratinocyte growth factor retards angiogenesis in cutaneous wounds. J Int Med Res 39:416–423
Takeshita S, Zheng LP, Brogi E, Kearney M, Pu LQ, Bunting S, Ferrara N, Symes JF, Isner JM (1994) Therapeutic angiogenesis: a single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J Clin Invest 93:662–670
Unger EF, Banai S, Shou M, Lazarous DF, Jaklitsch MT, Scheinowitz M, Correa R, Klingbeil C, Epstein SE (1994) Basic fibroblast growth factor enhances myocardial collateral flow in a canine model. Am J Physiol 266:H1588–H1595
Taniyama Y, Morishita R, Aoki M, Nakagami H, Yamamoto K, Yamazaki K, Matsumoto K, Nakamura T, Kaneda Y, Ogihara T (2001) Therapeutic angiogenesis induced by human hepatocyte growth factor gene in rat and rabbit hind limb ischemia models: preclinical study for treatment of peripheral arterial disease. Gene Ther 8:181–189
Tsurumi Y, Takeshita S, Chen D, Kearney M, Rossow ST, Passeri J, Horowitz JR, Symes JF, Isner JM (1996) Direct intramuscular gene transfer of naked DNA encoding vascular endothelial growth factor augments collateral development and tissue perfusion. Circulation 94:3281–3290
Feldman LJ, Steg PG, Zheng LP, Chen D, Kearney M, McGarr SE, Barry JJ, Dedieu JF, Perricaudet M, Isner JM (1995) Low-efficiency of percutaneous adenovirus-mediated arterial gene transfer in the atherosclerotic rabbit. J Clin Invest 95:2662–2671
Wilson JM (1995) Gene therapy for cystic fibrosis: challenges and future directions. J Clin Invest 96:2547–2554
Lynch JR, Fishbein M, Echavarria M (2011) Adenovirus. Semin Respir Crit Care Med 32:494–511
Morikawa O, Walker TA, Nielsen LD, Pan T, Cook JL, Mason RJ (2000) Effect of adenovector-mediated gene transfer of keratinocyte growth factor on the proliferation of alveolar type II cells in vitro and in vivo. Am J Respir Cell Mol Biol 23:626–635
Hall K, Blair ZM, Blair GE (2010) Unity and diversity in the human adenoviruses: exploiting alternative entry pathways for gene therapy. Biochem J 431:321–336
Amalfitano A, Chamberlain JS (1997) Isolation and characterization of packaging cell lines that coexpress the adenovirus E1, DNA polymerase, and preterminal proteins: Implications for gene therapy. Gene Ther 4:258–263
Koohsari H, Tamaoka M, Campbell HR, Martin JG (2007) The role of gamma delta T cells in airway epithelial injury and bronchial responsiveness after chlorine gas exposure in mice. Respir Res 8:21
Dun ZN, Zhang XL, An JY, Zheng LB, Barrett R, Xie SR (2010) Specific shRNA targeting of FAK influenced collagen metabolism in rat hepatic stellate cells. World J Gastroenterol 16:4100–4106
Khan A, Ashrafpour H, Huang N, Neligan PC, Kontos C, Zhong A, Forrest CR, Pang CY (2004) Acute local subcutaneous VEGF165 injection for augmentation of skin flap viability: efficacy and mechanism. Am J Physiol Regul Integr Comp Physiol 287:R1219–R1229
McFarlane RM, Heagy FC, Radin S, Aust JC, Wermuth RE (1965) A study of the delay phenomenon in experimental pedicle flaps. Plast Reconstr Surg 35:245–262
Rinker B, Fink BF, Barry NG, Fife JA, Milan ME (2010) The effect of calcium-channel blockers on smoking-induced skin flap necrosis. Plast Reconstr Surg 125:866–871
Huang N, Khan A, Ashrafpour H, Neligan PC, Forrest CR, Kontos CD, Pang CY (2006) Efficacy and mechanism of adenovirus-mediated VEGF-165 gene therapy for augmentation of skin flap viability. Am J Physiol Heart Circ Physiol 291:H127–H137
Fujihara Y, Koyama H, Nishiyama N, Eguchi T, Takato T (2005) Gene transfer of bFGF to recipient bed improves survival of ischemic skin flap. Br J Plast Surg 58:511–517
Honda T, Ishida K, Hayama M, Kubo K, Katsuyama T (2000) Type II pneumocytes are preferentially located along thick elastic fibers forming the framework of human alveoli. Anat Rec 258:34–38
Carvalho VL, Nunes MR, Da SE, Vieira CM, Gomes M, Casseb SM, Rodrigues SG, Nunes-Neto JP, Quaresma JA, Vasconcelos PF (2009) Genetic characterization of orthobunyavirus Melao, strains BE AR633512 and BE AR8033, and experimental infection in golden hamsters (Mesocricetus auratus). J Gen Virol 90:223–233
Kaczmarek A, Vandenabeele P, Krysko DV (2013) Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity 38:209–223
Auf DU, Krampert M, Kumin A, Braun S, Werner S (2004) Keratinocyte growth factor: effects on keratinocytes and mechanisms of action. Eur J Cell Biol 83:607–612
Xia YP, Zhao Y, Marcus J, Jimenez PA, Ruben SM, Moore PA, Khan F, Mustoe TA (1999) Effects of keratinocyte growth factor-2 (KGF-2) on wound healing in an ischaemia-impaired rabbit ear model and on scar formation. J Pathol 188:431–438
Richardson GD, Bazzi H, Fantauzzo KA, Waters JM, Crawford H, Hynd P, Christiano AM, Jahoda CA (2009) KGF and EGF signalling block hair follicle induction and promote interfollicular epidermal fate in developing mouse skin. Development 136:2153–2164
Acknowledgments
We thank Dr. Chen Li, Gong Li, and Fang Wen, who kindly provided pAd-KGF, Pa317, and HUVEC for this study.
Conflict of interest
The authors had no conflicts of interest to declare in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, X., Yu, M., Zhu, W. et al. Adenovirus-Mediated Expression of Keratinocyte Growth Factor Promotes Secondary Flap Necrotic Wound Healing in an Extended Animal Model. Aesth Plast Surg 37, 1023–1033 (2013). https://doi.org/10.1007/s00266-013-0200-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-013-0200-7