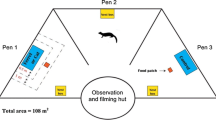
Abstract
Shelter competition is uncommon among social animals, as is the case among normally gregarious Caribbean spiny lobsters (Panulirus argus). However, healthy lobsters avoid sheltering with conspecifics infected by a lethal pathogenic virus, PaV1. These contradictory behaviors have implications for shelter use and survival, especially in areas where shelter is limited. In laboratory experiments, we tested shelter competition between paired healthy and diseased juvenile lobsters in shelter-limited mesocosms. Neither healthy nor diseased lobsters dominated access to shelters, but lobsters shared shelter less often when diseased lobsters were present relative to controls with two healthy lobsters. We hypothesized that exclusion of juvenile lobsters from shelter results in increased mortality from predation, especially for the more lethargic, infected individuals. Field tethering trials revealed that predation was indeed higher on infected individuals and on all tethered lobsters deprived of shelter. We then tested in mesocosm experiments how the contrasting risks of predation versus infection by a lethal pathogen influence shelter use. Lobsters were offered a choice of an empty shelter or one containing a diseased lobster in the presence of a predator (i.e., caged octopus) whose presence normally elicits shelter-seeking behavior, and these data were compared with a previous study where the predator was absent. Lobsters selected the empty shelter significantly more often despite the threat of predation, foregoing the protection of group defense in favor of reduced infection risk. These results offer striking evidence of how pathogenic diseases shape not only the behavior of social animals but also their use of shelters and risk of predation.




Similar content being viewed by others
References
Alzaga V, Vicente J, Villanua D, Acevedo P, Casas F, Gortazar C (2008) Body condition and parasite intensity correlates with escape capacity in Iberian hares (Lepusgranatensis). Behav Ecol Sociobiol 62:769–775
Behringer DC (2003) The ecological ramifications of density and disease in the Caribbean spiny lobster Panulirus argus. Dissertation. Old Dominion University
Behringer DC, Butler MJ IV (2006) Density-dependent population dynamics in juvenile Panulirus argus (Latrielle): the impact of artificial density enhancement. J Exp Mar Biol Ecol 334:84–95
Behringer DC, Butler MJ IV, Shields JD (2006) Avoidance of disease in social lobsters. Nature 441:421
Behringer DC, Butler MJ IV, Shields JD (2008) Ecological and physiological effects of PaV1 infection on the Caribbean spiny lobster (Panulirus argus Latreille). J Exp Mar Biol Ecol 359:23–33
Behringer DC, Butler MJ IV, Herrnkind WF, Hunt JH, Acosta CA, Sharp WC (2009) Is seagrass an important nursery habitat for the Caribbean spiny lobster, Panulirus argus, in Florida? NZ J Mar Freshw Res 43:327–337
Berger DK, Butler MJ IV (2001) Octopuses influence den selection by Caribbean spiny lobster. Mar Freshw Res 52:1049–1054
Bertelsen RD, Butler MJ IV, Herrnkind WF, Hunt JH (2009) Regional characterization of hard-bottom nursery habitat for juvenile Caribbean spiny lobster using rapid assessment techniques. NZ J Mar Freshw Res 43:299–312
Briones-Fourzán P, Lozano-Álvarez E, Negrete-Soto F, Barradas-Ortiz C (2007) Enhancement of juvenile Caribbean spiny lobsters: an evaluation of changes in multiple response variables with the addition of large artificial shelters. Oecologia 151:401–416
Butler MJ, Herrnkind WF (1997) A test of recruitment limitation and the potential for artificial enhancement of spiny lobster (Panulirus argus) populations in Florida. Can J Fish Aquat Sci 54:452–463
Butler MJ IV, Lear JA (2009) Habitat-based intraguild predation by Caribbean reef octopus (Octopus briareus) on juvenile Caribbean spiny lobster (Panulirus argus). Mar Ecol Prog Ser 386:115–122
Butler MJ IV, Stein RA (1985) An analysis of the mechanisms governing species replacements in crayfish. Oecologia 66:168–177
Butler MJ IV, Hunt JH, Herrnkind WF, Matthews T, Childress M, Bertelsen R, Sharp W, Field JM, Marshall H (1995) Cascading disturbances in Florida Bay, USA: cyanobacteria blooms, sponge mortality, and implications for juvenile spiny lobster Panulirus argus. Mar Ecol Prog Ser 129:119–125
Butler MJ IV, Herrnkind WF, Hunt JH (1997) Factors affecting the recruitment of juvenile Caribbean spiny lobsters dwelling in macroalgae. Bull Mar Sci 61:3–19
Butler MJ IV, MacDiarmid AB, Booth JD (1999) Ontogenetic changes in social aggregation and its adaptive value for spiny lobsters in New Zealand. Mar Ecol Prog Ser 188:179–191
Butler MJ IV, Behringer DC, Shields JD (2008) Transmission of Panulirus argus virus 1 (PaV1) and its effect on the survival of juvenile Caribbean spiny lobster. Dis Aquat Org 79:173–182
Childress MJ (2007) Comparative sociobiology of lobsters. In: Duffy JE, Thiel M (eds) Evolutionary ecology of social and sexual systems. Oxford University Press, New York, pp 271–293
Childress MJ, Herrnkind WF (2001) The guide effect influence on the gregariousness of juvenile Caribbean spiny lobsters. Anim Behav 62:465–472
Dolan TW III, Butler MJ IV (2006) The adaptive value of aggregation among juvenile Caribbean spiny lobster: an evaluation using individual-based modeling. J Crustac Biol 26:565–578
Eggleston DB, Lipcius RN (1992) Shelter selection by spiny lobster under variable predation risk, social conditions, and shelter size. Ecology 73:992–1011
Eggleston DB, Lipcius RN, Miller DL, Coba-Cetina L (1990) Shelter scaling regulates survival of juvenile Caribbean spiny lobster Panulirus argus. Mar Ecol Prog Ser 62:70–88
Fero K, Moore PA (2008) Social spacing of crayfish in natural habitats: what role does dominance play? Behav Ecol Sociobiol 62:1119–1125
Forcucci DM, Butler MJ IV, Hunt JH (1994) Growth and population dynamics of juvenile Caribbean spiny lobster, Panulirus argus, in Florida Bay, FL (USA). Bull Mar Sci 54:805–818
Foster WA, Treherne JE (1981) Evidence for the dilution effect in the selfish herd from fish predation on a marine insect. Nature 293:466–467
Frank SA (2003) Perspective: repression of competition and the evolution of cooperation. Evolution 57:693–705
Goldstein JS, Matsuda H, Takenouchi T, Butler MJ IV (2008) The complete development of larval Caribbean spiny lobster Panulirus argus (Latreille, 1804) in culture. J Crustac Biol 28:306–327
Herrnkind WF (1980) Spiny lobsters: patterns of movement. In: Cobb JS, Phillips BF (eds) The biology and management of lobsters: physiology and behavior (Vol. 1). Academic, New York, pp 349–407
Herrnkind WF, Butler MJ IV (1986) Factors regulating postlarval settlement and juvenile microhabitat use by spiny lobsters, Panulirus argus. Mar Ecol Prog Ser 34:23–30
Herrnkind WF, Butler MJ IV, Tankersley RA (1988) The effects of siltation on recruitment of spiny lobsters, Panulirus argus. Fish Bull 86:331–338
Herrnkind WF, Butler MJ IV, Hunt JH, Childress M (1997) Role of physical refugia: implications from a mass sponge die-off in a lobster nursery in Florida. Mar Freshw Res 48:759–769
Hixon MA, Beets JP (1993) Predation, prey refuges, and the structure of coral-reef fish assemblages. Ecol Monogr 63:77–101
Holbrook SJ, Schmitt RJ (2002) Competition for shelter space causes density-dependent predation mortality in damselfishes. Ecology 83:2855–2868
Hudson P, Greenman J (1998) Competition mediated by parasites: biological and theoretical progress. Trends Ecol Evol 13:387–390
Hudson PJ, Dobson AP, Newborn D (1992) Do parasites make prey vulnerable to predation? Red grouse and parasites. J Anim Ecol 61:681–692
Hughes WOH, Boomsma JJ (2004) Genetic diversity and disease resistance in leaf-cutting ant societies. Evolution 58:1251–1260
Hughes WOH, Eilenberg J, Boomsma JJ (2002) Tradeoffs in group living: transmission and disease resistance in leaf-cutting ants. Proc R Soc Lond B 269:1811–1819
Ives AR, Murray DL (1997) Can sublethal parasitism destabilize predator-prey population dynamics? A model of snowshoe hares, predators and parasites. J Anim Ecol 66:265–278
Kiesecker JM, Skelly DK (2001) Effects of disease and pond drying on gray tree frog growth, development, and survival. Ecology 82:1956–1963
Koprivnikar J, Forbes MR, Baker RL (2008) Larval amphibian growth and development under varying density: are parasitized individuals poor competitors? Oecologia 155:641–649
Lafferty KD, Morris AK (1996) Altered behavior of parasitized killifish increases susceptibility to predation by bird final hosts. Ecology 77:1390–1397
Lefcort H, Blaustein AR (1995) Disease, predator avoidance, and vulnerability to predation in tadpoles. Oikos 74:469–474
Lefèvre T, Lebarbenchon C, Gauthier-Clerc M, Misse D, Poulin R, Thomas F (2009) The ecological significance of manipulative parasites. Trends Ecol Evol 24:41–48
Lozano-Álvarez E, Briones-Fourzán P, Ramírez-Estévez A, Placencia-Sánchez D, Huchin-Mian JP, Rodríguez-Canul R (2008) Prevalence of Panulirus argus Virus 1 (PaV1) and habitation patterns of healthy and diseased Caribbean spiny lobsters in shelter-limited habitats. Dis Aquat Org 80:95–104
Lutermann H, Bennett NC (2008) Strong immune function: a benefit promoting the evolution of sociality? J Zool 275:26–32
Marcogliese DJ, Cone DK (1997) Food webs: a plea for parasites. Trends Ecol Evol 12:320–325
Marx J, Herrnkind WF (1985) Factors regulating microhabitat use by young juvenile spiny lobsters, Panulirus argus: food and shelter. J Crustac Biol 5:650–657
Mills DJ, Johnson CR, Gardner C (2008) Bias in lobster tethering experiments conducted for selecting low-predation release sites. Mar Ecol Prog Ser 364:1–13
Mintz JD, Lipcius RN, Eggleston DB, Seebo MS (1994) Survival of juvenile Caribbean spiny lobster: effects of shelter size, geographic location, and conspecific abundance. Mar Ecol Prog Ser 112:255–266
Møller AP, Erritzøe J (2000) Predation against birds with low immunocompetence. Oecologia 122:500–504
Mooring MS, Hart BL (1992) Animal grouping for protection from parasites – selfish herd and encounter-dilution effects. Behaviour 123:173–193
Murray DL (2002) Differential body condition and vulnerability to predation in snowshoe hares. J Anim Ecol 71:614–625
Ostfeld RS, Holt RD (2004) Are predators good for your health? evaluating evidence for top-down regulation of zoonotic disease reservoirs. Frontiers Ecol Environ 2:13–20
Peterson CH, Black R (1994) An experimentalist’s challenge: when artifacts of intervention interact with treatments. Mar Ecol Prog Ser 111:289–297
Peterson BJ, Chester CM, Jochem FJ, Fourqurean JW (2007) Potential role of sponge communities in controlling phytoplankton blooms in Florida Bay. Mar Ecol Prog Ser 328:93–103
Poulin R, Fitzgerald GJ (1989) Shoaling as an anti-ectoparasite mechanisms in juvenile sticklebacks (Gasterosteus spp). Behav Ecol Sociobiol 24:251–255
Pulliam HR (1973) On the advantages of flocking. J Theo Biol 38:419–422
Ratchford SG, Eggleston DB (1998) Size- and scale-dependent chemical attraction contribute to an ontogenetic shift in sociality. Anim Behav 56:1027–1034
Reinhard EG (1950) An analysis of the effects of a sacculinid parasite on the external morphology of Callinectes sapidus Rathbun. Biol Bull 98:277–288
Schaffer MW, Jensen DB, Hobbs DE, Gurevitch J, Todd JR, Schaffer MV (1979) Competition, foraging energetics, and the cost of sociality in 3 species of bees. Ecology 60:976–987
Schmidt KA, Ostfeld RS (2001) Biodiversity and the dilution effect in disease ecology. Ecology 82:609–619
Shields JD, Behringer DC (2004) A new pathogenic virus in the Caribbean spiny lobster Panulirus argus from the Florida Keys. Dis Aquat Org 59:109–118
Smith KN, Herrnkind WF (1992) Predation on early juvenile spiny lobsters Panulirus argus (Latreille): influence of size and shelter. J Exp Mar Biol Ecol 157:3–18
Söderbäck B (1994) Interactions among juveniles of two freshwater crayfish species and a predatory fish. Oecologia 100:229–235
Sousa WP (1991) Can models of soft-sediment community structure be complete without parasites? Am Zool 31:821–830
Steele MA (1999) Effects of shelter and predators on reef fishes. J Exp Mar Biol Ecol 233:65–79
Stevely JM, Sweat DE (1998) Survey of recovery of Florida Keys sponge populations following a widespread sponge mortality. Report No. MR252, Florida Sea Grant Extension Program. Tallahassee, Florida.
Temple SA (1987) Do predators always capture substandard individuals disproportionately from prey populations? Ecology 68:669–674
Thompson RM, Mouritsen KN, Poulin R (2005) Importance of parasites and their life cycle characteristics in determining the structure of a large marine food web. J Anim Ecol 74:77–85
Vorburger C, Ribi G (1999) Aggression and competition for shelter between a native and an introduced crayfish in Europe. Freshw Biol 42:111–119
Weiss HM, Lozano-Álvarez E, Briones-Fourzán P (2008) Circadian shelter occupancy patterns and predator-prey interactions of juvenile Caribbean spiny lobsters in a reef lagoon. Mar Biol 153:953–963
Acknowledgements
We thank J. Shields for advice regarding experiments and manuscript organization and two anonymous reviewers for their thorough critique of the manuscript. We also thank Goshen College for the use of their marine laboratory facilities and those who assisted in data collection including: A. Adamson, M. Kintzing, J. Lear, A. Mojica, C. Stall, and M. Dickson. Support for this project was provided by the National Science Foundation grant no. OCE-0452805 and the National Sea Grant College Program of the U.S. Department of Commerce’s National Oceanic and Atmospheric Administration (NOAA), Grant No. NA16RG-2195. The views expressed are those of the authors and do not necessarily reflect the view of these organizations.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Jennions
Rights and permissions
About this article
Cite this article
Behringer, D.C., Butler, M.J. Disease avoidance influences shelter use and predation in Caribbean spiny lobster. Behav Ecol Sociobiol 64, 747–755 (2010). https://doi.org/10.1007/s00265-009-0892-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-009-0892-5