Abstract
Variance in male reproductive success is expected to be high in sexually dimorphic mammals, even when it is modulated by the costs and benefits of group living. Here, we investigate the variance in reproductive success of male western gorillas (Gorilla gorilla), a highly dimorphic primate with long-term male–female associations, using 12.5 years of data collected at Mbeli Bai in northern Congo. Access to mates and offspring survival were both major sources of variance in male reproductive success. Males with larger harems had lower offspring mortality with no apparent reduction in female fertility or observed tenure length, so the size of harems did not seem to be limited by female feeding competition or by the risk of takeovers and infanticide by outsider males. The lower mortality in larger harems may reflect improved vigilance against predators, and females may cluster around males that enhance offspring survival. Thus, this study illustrates how a detailed analysis of the components of male reproductive success can shed light on the interrelated social and ecological aspects that affect it.
Similar content being viewed by others
Introduction
High variance in male reproductive success is assumed to provide a potential for sexual selection that can lead to sexual dimorphism (Darwin 1871; Andersson 1994). Sexual selection has been broadly defined as the portion of natural selection that arises from reproductive competition with members of the same sex, or more narrowly defined as the selection that arises from variance in the number of mates per male (Darwin 1871; Wade 1987; Clutton-Brock 2004). Sexual selection can involve competition to kill, suppress, or exclude rivals (intrasexual selection: Clutton-Brock et al. 1982; Kappeler 1997) as well as competition to attract mates (intersexual selection: Clutton-Brock and McAuliffe 2009; Jones and Ratterman 2009), and other mechanisms such as coercion (Andersson and Iwasa 1996; Pradhan and Van Schaik 2009).
Male reproductive success can be decomposed into different components such as the number of mates, the fertility of those mates, offspring survival, and longevity (Brown 1988). The most apparent manifestation of reproductive competition is often the variance in the number of mates per male, including variance between mating versus non-mating males (Wade and Shuster 2004). The other three components are not typically associated with sexual selection on males, but they can be influenced by reproductive competition in some species. Variance in female fertility could arise if more competitive males have preferential access to better resources or higher quality mates (Kelly 2008; Lee et al. 2008). Variance in offspring survival may arise if more competitive males provide better protection against infanticide by their rivals (Packer et al. 1988; Borries et al. 1999). Variance in longevity and the length of reproductive careers (tenure length) could arise if males are killed during mating competition, or if mortality increases due to costly dimorphic traits (Dunbar 1984; Clutton-Brock and Isvaran 2007). Components of reproductive success can also vary due to factors that are not directly related to reproductive competition, including environmental fluctuations or chance (Sutherland 1985; Coltman et al. 1999).
Components of male reproductive success can be related to each other, particularly among group-living polygynous species. Socio-ecological models predict that female fertility may decrease with group size due to greater feeding competition within groups, or it may increase due to improved competitiveness against other groups (e.g., Sterck et al. 1997). Offspring survivorship may increase with group size due to improved vigilance against predators, or it may decrease due to greater threats from infanticidal outsider males (Dunbar 1984; van Schaik 1989; Crockett and Janson 2000; Steenbeek and van Schaik 2001). Male longevity may be reduced in larger groups if increased mating competition increases the risk of mortality, leading to a potential tradeoff between having a few mates for a long time versus many mates for a short time (Dunbar 1984; Robinson et al. 2006). Alternatively, female fertility, offspring survival, and male longevity may all be greater in larger groups; if those groups tend to have higher quality males or occupy better habitats (McElligott et al. 2002; West and Packer 2002; Bro-Jorgensen and Durant 2003). Thus, it becomes important to understand how variance in male reproductive success in group-living animals depends upon the complex interplay of ecological, demographic, social, and life history factors (Pereira et al. 2000).
This paper examines the variance in reproductive success of male western gorillas using 12.5 years of data from Mbeli Bai, a large swampy forest clearing in the Nouabalé-Ndoki National Park of the Republic of Congo. Gorillas are the largest primates and among the most sexually dimorphic, with adult males (silverbacks) weighing twice as much as females (Leigh and Shea 1995; Smith and Jungers 1997). The extreme sexual dimorphism of gorillas is expected to coincide with high variance in male reproductive success (Darwin 1871; Leigh and Shea 1995), but the relative contributions from each component of male reproductive success are not well understood.
Western gorillas have a one-male mating system in which subordinate males typically emigrate to become solitary before reaching full maturity (Parnell 2002b; Robbins et al. 2004). In that regard, western gorillas differ from mountain gorillas where roughly half of maturing males remain in their natal group, and up to 40% of groups are multi-male (Kalpers et al. 2003; Stoinski et al. 2009). Up to 45% of silverbacks live alone or in non-breeding groups with other males, but it is unknown how many eventually acquire females (Parnell 2002b; Gatti et al. 2004). Harems of western gorillas form when females join a solitary male or non-breeding group, and they disintegrate when all females leave a harem holder or he disappears (ibid).
Unlike silverbacks, female gorillas transfer directly from one group to another during inter-group encounters, so they do not become solitary (Harcourt et al. 1976; Stokes et al. 2003). Females are believed to rely on males for protection against predators (leopards and humans) and infanticide (Stokes et al. 2003; Harcourt and Stewart 2007). Females do not transfer while lactating or pregnant, presumably due to the risk of infanticide if they joined a male who had not sired their offspring (Robbins et al. 2009a). Female western gorillas have not been reported to mate with subordinate or extra-group males (Mbeli Bai: Breuer, personal observation; Bai Hokou: Todd, personal communication; Mondika: Doran-Sheehy, personal communication), which is consistent with genetic analyses that assigned no paternity to those males (Mondika: Bradley et al. 2004).
The potential for sexual selection in gorillas reflects a combination of intrasexual selection, intersexual selection, and other mechanisms. As expected under intrasexual selection, male–male competition during inter-group encounters can involve intense physical fights that are occasionally fatal (Watts 1989; Robbins 2003; Jeffery et al. 2007). However, there is no clear winner during many encounters between males, so female transfers during those encounters could be considered mate choices that lead to intersexual selection. Dominant males may limit the emigration of their females through coercion and herding (Sicotte 1993; Levréro 2005; Robbins 2009).
Essentially all adult females reproduce in this population, so we predict that much of the variance in male reproductive success will arise from differences in the ability to acquire and retain those females. We predict that differences in female fertility will be a relatively minor source of variance in male reproductive success because competition for resources is considered to be low (Stokes et al. 2003). We predict that predation and infanticide will create variance in offspring survival that reflects the ability of males to protect their infants. We are unable to confirm the cause of offspring mortality, but we propose that infanticide can occur throughout all ages of infancy, whereas predation by leopards may become more likely as offspring approach weaning age and become more independent from their mothers. This study was not long enough to assess the longevity of western gorillas, but we used the observed tenure length to begin examining the potential tradeoff between having a few mates for a long time versus many mates for a short time. We examine the potential influences upon each component of reproductive success and discuss the implications for selection within this population and others.
Methods
Study population
We studied western gorillas at Mbeli Bai, a large (12.9 ha) swampy forest clearing in the southwest of the Nouabalé-Ndoki National Park, Republic of Congo, that is characterized by superabundant aquatic herbs (for details about the study site, see Parnell 2002a, b; Stokes et al. 2003; Breuer 2008). Four principal investigators (C.O.: 1995–1997; R.J.P.: 1997–1999; E.J.S.: 1999–2002; T.B.: 2002–2007) and assistants monitored the gorillas visiting the clearing between February 1995 and July 2007 (3,957 days, average of 9.1 h/day) with an absence from the clearing for 2 months in 1997 due to civil unrest in the country. Gorillas were observed with help of telescopes and binoculars from an observation platform that provides almost 100% visibility from the edge of the clearing. Due to the unrestricted visibility in the forest clearing, solitary males could be observed just as easily as breeding groups, so our study should provide an unbiased estimate of non-mating males (Parnell 2002b; Gatti et al. 2004). Identification of gorillas was based upon features such as shape of brow-ridges, ears, nose-prints, and pelage (Parnell 2002a). We did not use group membership as criterion for identification of individuals. After a period of approximately 3 months training with the previous investigator, it was generally possible to identify all gorillas with the help of photos, videos, and “identity cards” that highlighted the distinguishing features of each individual. We tried to have at least two observers on the platform at any time, who discussed the identity of gorillas to further reduce any risk of inter-observer variability. In addition, TB reviewed more than 200 h of video footage and confirmed the identifications of prior investigators (Breuer et al. 2009).
Monitoring demographic changes (transfer, birth, and mortality dates)
Mbeli Bai has an open population of gorillas. Since each gorilla does not visit the clearing every day, there are gaps of observation, and we had to estimate the dates of birth, death, and dispersal. Some gorillas have been observed within 1–2 days after their birth, as confirmed because their group had just been seen without them. When gorillas were first observed beyond that age, their birthdates were estimated by comparing their morphological and behavioral characteristics with other gorillas whose age was already determined (Parnell 2002a; Nowell 2005; Breuer et al. 2009). We believe the precision of those birthdates is within a few weeks for most gorillas who were first observed as infants, and within 1–2 years for gorillas who were first observed as they approached adulthood. Adulthood was defined to begin at age ten for females and 18 for males, which is when they begin to reproduce (Breuer et al. 2009).
Dispersal dates were typically determined as the midpoint between visits of the group of origin and the group of destination (Stokes et al. 2003). When a gorilla stopped visiting the Bai, we were generally unable to confirm whether it had died or dispersed. If the missing gorilla had been a harem holder, we assumed that he died. This was a plausible assumption because in nine out of 15 cases, the silverback had signs of serious injuries or was extremely thin in previous visits before disappearance. After disappearing from their group, harem holders were almost never seen again, whereas most maturing subordinate males were still seen following their emigration to become solitary. Dates of disappearance were typically determined as the midpoint between the last time an individual was observed and the first time that the group was seen without him.
It is unlikely that unweaned infants (<4 years) could survive without their mothers (Nowell and Fletcher 2007; Breuer et al. 2009), so if they disappeared before that age, we assumed that they had died. Due to the nature of the study and intermittent observations, we might have missed a few infants who were born and died before we saw them, which would lead to an underestimation of female fertility and offspring mortality. As evidence of such missed infants, the apparent mortality rate was lower than in mountain gorillas during the first 6 months of infancy, despite higher mortality throughout the rest of infancy (Fig. 1). Nonetheless, the results of this study did not change appreciably when we limited the analyses to groups that made at least 12 visits per year, with no observation gaps longer than 3 months. Therefore, we present the results that do not exclude groups which were observed less frequently.
Measures of male reproductive success
Our first two measures of male reproductive success involve a subtle distinction between harem size (H) and the number of mates per male (N MATE). Both parameters are based upon the number of adult females that were with a male, but whereas our calculations of harem size exclude males when they have no harem, those males are assigned a value of zero in calculations of N MATE (e.g., Wade and Shuster 2004). For example, if we observed one harem with two females, another harem with four females, and one solitary male, then the mean harem size (M HAREM) would be \( \left( {2 + 4} \right)/2 = 3 \), and the mean number of mates per male (M MATE) would be \( \left( {2 + 4 + 0} \right)/3 = 2 \).
In addition to the mean for both measures of male reproductive success, we also calculated the variance and the standardized variance (variance/mean2). The standardized variance in reproductive success has been defined as the “opportunity for selection”, which reflects an upper limit to the strength of natural selection including sexual selection (Crow 1958; Trail 1985; Downhower et al. 1987; Shuster and Wade 2003; Mills et al. 2007). The standardized variance in the number of mates per male (I MATE) has been defined as the “opportunity for sexual selection” (Wade 1979; Wade and Arnold 1980; Jones 2009). The variance in the number of mates per male (V MATE) includes variance among harem-holding males as well as variance between harem-holding versus non-harem-holding males. The proportion of variance that arose between harem-holding males versus non-harem-holding males is indicated by the R 2 value from an analysis of variance, in which the dependent variable was the value of N MATE for each male, and the category variable indicated whether he was a harem holder.
We examined both H and N MATE from two perspectives: “snapshots” of the population on a particular day, as well as longitudinal analyses throughout the adulthood of each male. The snapshot calculations used the actual number of adult females that were with each male on the day of the snapshot. For example, on January 1st 1996, the study population contained harems with 5, 5, 7, 7, 8, 8, 8, 9, 9, 11, and 12 females, for a average harem size of 3.1 females. We performed a separate snapshot calculation for the first day of each year in the study. There is a continuous male–female association in harems of western gorillas; hence, there is no reason to expect different results on other days of the year.
The longitudinal analyses used overall values from throughout the observations of each male. For example, if one male had an average harem size of 2.1 females throughout our observations of his adulthood and another male had an average harem size of 3.3 females throughout our observations of his adulthood, then the average harem size among those two males would be \( \left( {2.1 + 3.3} \right)/2 = 2.7 \) females per male. For all other measures of reproductive success (below), we performed only the longitudinal analyses based on the overall observations of each male.
Our next two measures of reproductive success for each male were the average birth rate of his females (BR) and the proportion of his offspring that survived to a specified age (SV). The average birth rate of his females equaled the number of births that occurred in his group during the study, divided by the number of female-years observed in that group (e.g., see Robbins et al. 2007). We used three criteria for offspring survival: ages one, three, and four. Age four was chosen because it is the typical weaning age in the Mbeli Bai population (Nowell and Fletcher 2007; Breuer et al. 2009). Studies of reproductive success often consider offspring survival until their age of first reproduction (Brown 1988; Strassmann and Gillespie 2003), but the weaning age should be a reasonable approximation for this study because subsequent mortality until the age of first reproduction is minimal for mountain gorillas (Gerald 1995). Mountain gorillas are typically weaned at a younger age than western gorillas (Watts 1991), so age three is often used as the criterion for offspring survival in that population (Bradley et al. 2005), and we present such results for comparative purposes. All analyses involving infant survival were limited to dates in which it could be fully evaluated, which led to differences in sample sizes among some analyses (e.g., for survival to age four, we excluded infants born during the last 4 years of the study). Therefore, we also performed analyses based on survival to age one because it enabled us to use a greater proportion of the dataset.
The siring rate of each male (SR) was calculated as the number of offspring born in his group that survived to a specified age, divided by the number of years that he was observed as an adult. Again, we used three criteria for offspring survival: ages one, three, and four; and the analyses were limited to dates in which it could be fully evaluated. For example, if a male was observed for 5 years before July 2003 (thus excluding the last 4 years of the study) and three of the offspring that were born in his group during those years survived to reach age four, his rate of siring offspring that survived to reach age four would be 3/5 = 0.60 surviving offspring per male-year. For the siring rate at each age criterion, we report the mean, standard deviation, and the standardized variance.
The siring rate for each male equals the product of three components: his longitudinal values for N MATE, BR, and SV. We used the calculations from Brown (1988) to determine how each component contributes to the variance among breeders (see Arnold and Wade 1984a, b; Spong et al. 2008 for alternative approaches). Specifically, we report the G′ values from upward partitioning as presented in Table 27.1 of Brown (1988), although that example uses the method with a different set of components (see Clutton-Brock 1988; Brown and Alexander 1991; Coltman et al. 1999; Setchell et al. 2005; Hodge et al. 2008 for additional descriptions of the approach). The first step in our calculations was to normalize the data for each component, by dividing the value for each male by the mean of the component among all males. For example, if Y n is the array of values for N MATE (one value for each male) and X n is the array of normalized values, then X n = Y n / mean (Y n). Next, we calculated the product of all three normalized components for each male (i.e., X n × X b × X s, in which X b and X s are the arrays of normalized values for BR and SV). Then, we calculated the variance for each of the normalized components and their product. For example,
in which “Var” signifies the variance among males for the value in parentheses. G b and G s were calculated with an analogous form of Eq. 1. Finally, we multiplied each of the G values by (100 / G nbs) to produce the G′ values. The G′ value for each component indicates how much of the variance in the siring rate arose from the variance of the component by itself, as if each male had the mean value for the other components. We used the bootstrap procedure from Brown (1988) to estimate the 95% confidence intervals of the G′ values.
In addition to examining the variance in the siring rate among breeders, we also calculated the variance among all males:
in which “SR a” is the array of siring rates for all males, “SR b” is the siring rates for all breeders, and “p” is the proportion of males that were breeders (Brown 1988). The term in the first brackets is the contribution due to variance among breeders. The term in the second brackets is the contribution due to the difference between breeders versus non-breeders. We report the proportion of the total variance that was due to the difference between breeders versus non-breeders (ibid).
The lifetime reproductive success of each male will equal his siring rate multiplied by his longevity. This study was not long enough to measure the longevity of western gorillas, but we made some preliminary assessments using the observed tenure length as a proxy (e.g., see Lawler 2007). The observed tenure length was calculated as the interval between the first and last date that a silverback was observed as a harem holder. This data includes tenures that were ongoing at the beginning or the end of the study, and the observed tenure is the portion of those tenures that was observed. We limited the analysis to harem holders that could have sired offspring surviving to weaning age to reduce the bias towards males that were only briefly monitored. Three silverbacks temporarily lost all of their females, but we still included time in the non-breeding group as part of their observed tenure because such intervals can be important for infant survivorship if the group still contains offspring of the silverback.
The calculations for harem size and the number of mates per male were performed using Systat 11 (2004, SYSTAT Software Inc., Richmond, CA, USA). The Brown (1988) partitioning calculations were performed in an Excel spreadsheet. Spearman rank correlations were calculated to examine relationships among the three components with an Excel macro and are either exact (when n ≤ 9) or based on 10,000 permutations (Mundry and Fischer 1998). We used the same macro to separately investigate the correlation of tenure length with male reproductive success. Unless otherwise stated, values are given as mean ± standard deviation.
Results
Harem size and the number of mates per male (N MATE)
Harem size is often reported from census data, so for comparative purposes, we examined “snapshots” of our study population on the first day of each year (Table 1). From 1996 to 2007, the mean harem ranged from 2.9 to 4.3 adult females per harem-holding male. The standardized variance in harem size ranged from 0.28 to 0.47, which is higher than only 33–55% of the comparable values in a wide-ranging survey of crustaceans, insects, amphibians, reptiles, birds, and mammals (Wade and Shuster 2004). From that perspective, the standardized variance in harem size for western gorillas does not seem exceptionally large in comparison with other species.
Under strong sexual selection, a large portion of the variance in access to mates is predicted to arise from the difference between harem holders versus non-harem holders, but the latter category of males is often difficult to quantify accurately (Wade and Shuster 2004). At Mbeli Bai, solitary males can be detected just as easily as groups, so we examined their contribution to the overall variance among all males. On the first day of each year from 1996 to 2007, the average number of mates per male (M MATE) ranged between 1.9 and 2.8 adult females. (M MATE differs from the mean harem size because it includes males when they had no mates). Those snapshot values of M MATE are equivalent to the adult sex ratio, and they partly reflect the difference in the ages at which each sex is considered an adult (age ten for females versus 18 for males). The standardized variance in N MATE equaled 1.1 ± 0.2 (see the rows for mean and standard deviation of I MATE in Table 1). On average, 34% of adult males were non-harem holders, and 48% of the variance in N MATE arose between harem holders versus non-harem holders. Thus, our calculations support theoretical predictions that non-harem holders can account for much of the variance in access to mates.
The snapshot calculations from the first day of each year may either overestimate or underestimate the variance in the lifetime reproductive success among males, depending upon how N MATE typically changes throughout adulthood (Wade and Shuster 2004). To assess such potential bias, we repeated the calculations by using the longitudinal average of N MATE for each male, taken from all observations of the male rather than a snapshot value (Fig. 2). Whereas 34% of adult males were typically non-harem holders during the snapshot calculations of a single year, 12 of 41 males (29%) were never harem holders throughout our observations. Those on-going non-harem holders accounted for 36% of standardized variance in N MATE, which equaled 1.2 in the dataset with all males. Next, we repeated the calculations with the 11 males who were observed for at least 10 years or their complete adulthood. In that data subset, the proportion of non-harem holders dropped to 18% (two males who were either solitary or living in non-breeding groups for at least 12 years of observations). The standardized variance in N MATE was 1.0, with 40% of that variance coming from non-harem holders. So although the snapshot calculations may have overestimated the proportion of males who will never become harem holders, they seem to provide reasonable estimates for the long-term variance in N MATE.
Observed frequency distribution for the number of males with each value of N MATE. For this longitudinal analysis, the number of mates for each male (N MATE) was calculated as the average value from the first day of all months that he was observed as an adult. Whereas the average harem size would exclude months when a male had no harem, this calculation includes a value of zero for those months. The first bar shows 12 males who were never harem holders. The second bar shows seven males whose average number of mates was less than one, even though they were harem holders at some point during the study
Female fertility and offspring survival
To assess whether harem sizes might be limited by feeding competition among females, we looked for lower female fertility in larger harems. Among the 29 harem holders, the average female fertility was 0.16 ± 0.14 offspring per female-year. Males with larger harems had significantly higher female fertility, which is in the opposite direction of predictions for female feeding competition within groups (r S = 0.48, p = 0.011). However, those values may be biased because female fertility was zero for nine males (31%) who were observed only briefly as harem holders (e.g., because all of the females in the group were already nursing when the study began, and then the male died before any of those females were ready to give birth again). After excluding those nine males, female fertility was 0.24 ± 0.10 offspring per female-year, and it was not significantly correlated with harem size (r S = −0.11, p = 0.64). Thus, we did not find evidence that feeding competition among females has reduced their fertility in larger harems.
To examine whether the optimal harem size might be influenced by predation or infanticide, we looked for correlations between harem size and offspring survival at each age criterion. Predation is considered more likely to afflict smaller groups and older infants, whereas infanticide is more likely for larger groups and all infants (e.g., van Schaik 1983; Crockett and Janson 2000). As we increased the criteria for offspring survival from age one to four, the average offspring survival per male dropped from 82% to 39%. Males with larger harems had significantly higher offspring survival to age four, but the correlations were not significant for ages one and three (Table 2). Those results are more consistent with the predictions for predation than infanticide, and they give no indication that the optimal harem size might be limited by the risk of offspring mortality.
Siring rate
Having analyzed the variance in N MATE, female fertility, and offspring survival, we next used the Brown (1988) partitions to examine the variance in their product: the rate that each male sired offspring who survived to each age criterion. As we increased the criteria for offspring survival from age one to age four, the mean siring rate among all males declined from 0.34 to 0.20 surviving offspring per male-year, and the standardized variance increased from 1.8 to 3.5 (Table 3). The proportion of non-breeders remained near 50%, but their contribution to the variance in siring rate dropped from 51% to 25%. Therefore, as offspring survival increased the variance in siring rate among breeding males, the relative importance of non-breeders declined.
When the siring rate was based upon offspring survival to age one, N MATE accounted for 62% of its variance among breeders (Table 2), which was larger than the contributions from female fertility (49%) and offspring survival (15%). Those G′ values indicate (for example) that if each male had the mean values for female fertility and offspring survival and the observed variation in N MATE was the only difference among males, then the variance in the siring rate would be only 62% of its observed value. The G′ values do not add up to 100% due to covariance among the components. Males with more mates had significantly higher siring rates when based on all three age criteria for offspring survival. However, when the criterion for offspring survival was extended to ages three and four, N MATE accounted for only 43% and 26% of the variance in the siring rate. Offspring survival became the largest source of variance in the siring rate, explaining 54% of that variance among breeders (Fig. 3). Males with higher offspring survival had significantly higher siring rates at all three age criteria.
Estimated bootstrap sampling distributions for variance contributions to the rate of siring offspring that survived to reach age four. The G′ values were calculated from upward partitioning as presented in Table 27.1 of Brown (1988). The approximate 95% confidence intervals were 7–53% for the number of mates per male (N MATE, a), 5–44% for female fertility (b), and 30–91% for offspring survival (c). For each graph, the numbers on the x-axis indicate the minimum value for G′ that was tallied in each bar. The y-axis shows the proportion of the 10,000 bootstrap iterations that predicted each G′ value. For example, 7% of the iterations predicted a G′ value for N MATE that was at least 0% but less than 10% (the first bar in a)
zHypothetically, the large contribution from variance in offspring survival could be an artifact of this study because it contains some males that have died and others that have not. Offspring mortality is expected to increase when a silverback dies in a one-male group because his infants become more vulnerable to predators and infanticide by other males. Accordingly, nine of 11 infants (82%) died following the death of the silverback in their group, versus only 31 of 64 infants (48%) when the silverback remained alive (Fisher’s exact test: p = 0.053). Four of the nine deceased infants were less than 6 months old, one was between 2.0 and 2.5 years old, and the other four infants were between 3.0 and 3.5 years old (Fig. 1). Overall, infant mortality was similar to one-male groups of mountain gorillas, which face a higher infanticide risk than their multi-male groups (Table 4).
To test whether silverback mortality was responsible for the influence of offspring survival upon variance in siring rates, we repeated the Brown partitioning while excluding males who had infants when they died. In that analysis, offspring survival accounted for 57% of the variance in siring rate, which is essentially identical to the corresponding value of 54% from the full dataset (both analyses are based on offspring survival to age four). Offspring survival also accounted for 56% of the variance in siring rate in the data subset of males with long/complete observations, where the death of a silverback would affect a smaller proportion of his observed offspring. Thus, the large contribution of offspring survival to the variance in siring rate does not seem to be an artifact of whether males had unprotected infants during the study.
Observed tenure length
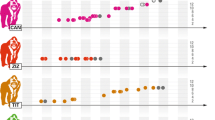
Males with higher siring rates will not necessarily have higher lifetime reproductive success, if they have shorter reproductive lifespans due to the increased competition of defending a larger harem. To begin investigating this possibility, we looked for a potential tradeoff between their observed tenure length and their average harem size. The observed tenure length has averaged 5.9 ± 3.7 years (range, 0.8–12.3; n = 24 tenures). Males with a higher average harem size had significantly longer observed tenures, which is in the opposite direction of any tradeoff between having a few mates for a long time versus many mates for a short time (r S = 0.58, p = 0.0029, n = 24 tenures). In addition, the observed tenure length was positively correlated with the siring rate to age four and the cumulative number of weaned offspring per male (siring rate, r S = 0.74, p < 0.001; number of offspring, r S = 0.78, p < 0.001; n = 24 tenures). Thus, males with longer observed tenures had higher reproductive success.
Discussion
The standardized variance was 1.0–1.2 for the number of mates per male (N MATE) versus 1.8–3.2 for the rates of siring offspring that survived to reach ages one to four. The difference between those two measurements mainly reflects variance in offspring survival, which became increasingly important as we incorporated a greater proportion of infancy into the analyses. Thus, access to mates and offspring survival were both major sources of variance in male reproductive success, while differences in female fertility were less important. Preliminary results showed higher reproductive success for males with longer observed tenures as harem holders.
Harem size and number of mates per male
Males with larger harems had higher siring rates, which is consistent with higher reproductive success for such males in mountain gorillas (Watts 2000; Robbins and Robbins 2005; Bradley et al. 2005) and many other species such as red deer, elephant seals, and Soay sheep (Clutton-Brock et al. 1988; Le Boeuf and Reiter 1988; Coltman et al. 1999). Larger harems are expected to lead to higher male reproductive success unless the benefits of increased mating opportunities are offset by costs such as increased feeding competition (Sterck et al. 1997), greater infanticide risk (Crockett and Janson 2000; Steenbeek and van Schaik 2001), or shorter tenures (Dunbar 1984; Packer et al. 1988). Similar to gorillas, the group sizes of Thomas’s langurs (Presbytis thomasi) are determined by female dispersal, which is related to the ability of a male to protect females and offspring (Steenbeek et al. 2000). In this study, males with larger harems had lower offspring mortality with no apparent reduction in female fertility or observed tenure length (see below), so the factors that are limiting harem size remain unclear.
Up to 58% of the variance in access to mates arose from the difference between harem holders versus non-harem holders (Table 1), which is consistent with theoretical predictions that such differences can be important (Wade and Shuster 2004). Single season assessments of the variance in harem size will underestimate the contribution of non-harem holders if males typically become more successful as they age, and it will overestimate that contribution if aging males typically become less successful (ibid). Rather than consistently increasing or decreasing with age, however, male competitive ability has been considered to follow an inverted-U shaped trajectory in many species (e.g., Alberts et al. 2003; Robinson et al. 2006). A nonlinear pattern may explain why the single season estimates of variance were fairly consistent with longer-term calculations in this study.
Female fertility
Female fertility is often lumped together with harem size to make the siring rate a single component of male reproductive success (e.g., Brown 1988), but separating the two parameters can help to investigate evidence of feeding competition among or within groups. Of the parameters that we examined, female fertility had the least influence upon variance in male siring rates. This is not surprising because fertility is mainly expected to depend upon food availability, and male gorillas do not seem to compete for resources for their group (Sicotte 1993; Robbins and Sawyer 2007; but see Bermejo 2004). In that regard, western gorillas differ from species such as chimpanzees whose males defend territories, and black and white colobus monkeys whose males are the main participants in feeding competition between groups (Fashing 2001; Williams et al. 2004; Harris 2006). Like those species, western gorillas have some clumped foods, but they also feed on more evenly distributed foliage, which may be sufficiently abundant to minimize competition among groups (Stokes 2004). More quantitative ecological comparisons would be needed to fully determine why those species differ from western gorillas and to determine whether female fertility is a greater source of variance in their male reproductive success.
Female fertility was not significantly lower in larger groups, which is consistent with other studies that found little or no influence of feeding competition upon the birth rates, inter-birth intervals, offspring survival, and dispersal patterns of female gorillas (Watts 1990; Stokes et al. 2003; Robbins et al. 2007, 2009b). Similarly, studies of other folivorous species have not consistently found evidence for greater feeding competition within larger groups, possibly because females may reduce the impact of such competition by increasing group spread, or because their food is relatively abundant, and/or because larger groups may have better habitat (Isbell 1991; Janson and Goldsmith 1995; Gillespie and Chapman 2001; Borries et al. 2008).
Offspring survival
As we extended our criterion for offspring survival from age one to age four, it became the largest source of variance in siring rates among breeders (Table 2). At all age criteria, higher offspring survival led to significantly higher male siring rates. Offspring survival was higher in larger groups, though the relationship was significant only at age four. We consider three potential explanations that are not mutually exclusive. Firstly, females may prefer larger groups to reduce the risk of predation (Hamilton 1971; Elgar 1989; Caro 2005). Larger groups can detect predators more quickly, they may overwhelm a predator by mobbing or scattering, and their individuals may be safer due to dilution effects (ibid). If the risk of predation declines in larger groups, it could amplify the strength of selection for sexually dimorphic traits that enable males to acquire females, because males with more mates would have higher offspring survival. Such a conclusion must be considered tentative, however, because there are no confirmed cases of predation on infant gorillas in this population.
Secondly, females may aggregate around silverbacks with “good genes” (Zahavi 1975). Such female mate choices can lead to intersexual selection for extravagant secondary sexual traits (Andersson 1994). Harem size has been positively correlated with sexually dimorphic traits in comparative studies of pinnipeds, primates, and ungulates, although those traits may reflect a combination of both intersexual and intrasexual selection (Alexander et al. 1979; Lindenfors et al. 2002; Vanpé et al. 2008). Similarly, male western gorillas with larger sagittal crests and larger gluteal muscles have larger harems, higher offspring survival, and higher rates of siring offspring that reach weaning age (Breuer 2008; Caillaud et al. 2008). If silverbacks with those traits have good genes, they could sire higher quality offspring with increased survivorship (Moller and Alatalo 1999; Byers and Waits 2006). Conversely, extravagant sexual traits are often predicted to reduce survivorship while increasing the siring rates of the few males who survive (Lande 1981; Oufiero and Garland 2007).
Thirdly, females may aggregate around males who are good protectors. Female gorillas do not rely solely on “safety in numbers” to reduce the risk of predation to themselves and their offspring; they rely on a silverback who is twice their size with larger canines (Leigh and Shea 1995; Smith and Jungers 1997; Thoren et al. 2006). The dominant male plays the primary role in confronting threats from both predators and potentially infanticidal outsider males (Harcourt and Stewart 2007), so selection is likely to favor sexually dimorphic traits that enhance his ability to succeed in that role. For example, crest size may correlate with biting strength (Parnell 2002a; see also Anderson et al. 2008 for other correlates of biting power). Confronting predators is not typically considered an aspect of male mating competition, so it would not even fit within relatively broad definitions for sexual selection (Clutton-Brock 2004). In contrast, infanticide is often considered part of intrasexual selection because it can enable a male to reproduce more quickly with the mother of the infant that he kills (i.e., the sexual selection hypothesis: Hrdy 1974; van Schaik and Janson 2000).
There is still no proof of infanticide in this population, but several suspected cases have been reported including direct observations of one fatally wounded infant (Stokes et al. 2003). More conclusive evidence of infanticide has been reported for mountain gorillas, particularly after the silverback dies in a one-male group (Watts 1989). Similarly, when silverbacks in this study had infants at the time of their death, 82% of those infants died shortly thereafter. In this respect, gorillas are similar to lions (Panthera leo), which also have infanticide and year-round harem defense, and in which offspring survival is an important component of male reproductive success (Packer et al. 1988). Infanticide is considered a major influence upon the social systems of many species (Sterck et al. 1997; van Schaik and Kappeler 1997; van Schaik and Janson 2000). The potential for infanticide can vary considerably among species, depending on the probability that a mother will encounter males who could not have sired her offspring, and whether her inter-birth interval will be shorter if her infant dies, and whether the infanticidal male would be likely to sire her next offspring (Harcourt and Greenberg 2001; van Schaik et al. 2004; Pradhan and van Schaik 2008; Boyko and Marshall 2009). Further study is needed to determine whether differences in the potential for infanticide contribute to differences in sexual dimorphism among species (Mitani et al. 1996; Lindenfors 2002; Thoren et al. 2006).
Observed tenure length
Silverbacks with larger harems had longer observed tenure lengths, so we found no evidence of a potential tradeoff between having a few mates for a long time versus many mates for a short time. In some species, male tenure is shorter in groups containing a large number of females (Packer et al. 1988; Steenbeek et al. 2000), which is probably due to the increased reproductive effort and higher risk of takeover by other males. In contrast, a lack of such a negative relationship (or the existence of a positive relationship) might be due to higher quality males attracting more females and simultaneously being able to maintain a long reproductive lifespan (McElligott and Hayden 2000). In this study, males with longer observed tenures had higher siring rates and a higher number of weaned offspring (see also Packer et al. 2001). Thus, the benefits of a long adulthood may be amplified because a longer tenure enables a silverback to fully protect a greater proportion of his offspring, which can further affect female preferences for younger and stronger males (Steenbeek et al. 2000). If so, then we expect that longevity and reproductive tenure may be a major source of variance in the lifetime reproductive success of male western gorillas, but it will not negate the importance of harem size or offspring survival as quantified by this study.
References
Alberts SC, Watts HE, Altmann J (2003) Queuing and queue-jumping: long-term patterns of reproductive skew in male savannah baboons, Papio cynocephalus. Anim Behav 65:821–840
Alexander RD, Hoogland JL, Howard RD, Noonan KM, Sherman PW (1979) Sexual dimorphism and breeding systems in pinnipeds, ungulates, primates and humans. In: Chagnon NA, Irons W (eds) Evolutionary biology and human social behavior: an anthropological perspective. Duxbury Press, Scituate, pp 402–435
Anderson RA, McBrayer LD, Herrel A (2008) Bite force in vertebrates: opportunities and caveats for use of a nonpareil whole-animal performance measure. Biol J Linn Soc 93:709–720
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Andersson M, Iwasa Y (1996) Sexual selection. Trends Ecol Evol 11:A53–A58
Arnold SJ, Wade MJ (1984a) On the measurement of natural and sexual selection—applications. Evolution 38:720–734
Arnold SJ, Wade MJ (1984b) On the measurement of natural and sexual selection—theory. Evolution 38:709–719
Bermejo M (2004) Home-range use and intergroup encounters in western gorillas (Gorilla g. gorilla) at Lossi Forest, north Congo. Am J Primatol 64:223–232
Borries C, Launhardt K, Epplen C, Epplen JT, Winkler P (1999) Males as infant protectors in Hanuman langurs (Presbytis entellus) living in multimale groups—defence pattern, paternity and sexual behaviour. Behav Ecol Sociobiol 46:350–356
Borries C, Larney E, Lu A, Ossi K, Koenig A (2008) Costs of group size: lower developmental and reproductive rates in larger groups of leaf monkeys. Behav Ecol 19:1186–1191
Boyko RH, Marshall AJ (2009) The willing cuckold: optimal paternity allocation, infanticide and male reproductive strategies in mammals. Anim Behav 77:1397–1407
Bradley BJ, Doran-Sheehy DM, Lukas D, Boesch C, Vigilant L (2004) Dispersed male networks in western gorillas. Curr Biol 14:510–513
Bradley BJ, Robbins MM, Williamson EA, Steklis HD, Steklis NG, Eckhardt N, Boesch C, Vigilant L (2005) Mountain gorilla tug-of-war: silverbacks have limited control over reproduction in multimale groups. Proc Natl Acad Sci U S A 102:9418–9423
Breuer T (2008) Male reproductive success in wild western gorillas (Gorilla gorilla). PhD thesis, University of Leipzig, Leipzig
Breuer T, Breuer-Ndoundou Hockemba M, Olejniczak C, Parnell RJ, Stokes EJ (2009) Physical maturation, life-history classes and age estimates of free-ranging western gorillas—insights from Mbeli Bai, Republic of Congo. Am J Primatol 71:106–119
Bro-Jorgensen J, Durant SM (2003) Mating strategies of topi bulls: getting in the centre of attention. Anim Behav 65:585–594
Brown D (1988) Components of lifetime reproductive success. In: Clutton-Brock T (ed) Reproductive success. University of Chicago Press, Chicago, pp 439–453
Brown D, Alexander N (1991) The analysis of the variance and covariance of products. Biometrics 47:429–444
Byers JA, Waits L (2006) Good genes sexual selection in nature. Proc Natl Acad Sci U S A 103:16343–16345
Caillaud D, Levrero F, Gatti S, Menard N, Raymond M (2008) Influence of male morphology on male mating status and behavior during interunit encounters in western lowland gorillas. Am J Phys Anthropol 135:379–388
Caro T (2005) Antipredator defenses in birds and mammals. University of Chicago Press, Chicago
Clutton-Brock T (1988) Reproductive success. University of Chicago Press, Chicago
Clutton-Brock TH (2004) What is sexual selection? In: Kappeler PM, van Schaik C (eds) Sexual selection in primates: new and comparative perspectives. Cambridge University Press, New York, pp 24–36
Clutton-Brock TH, Isvaran K (2007) Sex differences in ageing in natural populations of vertebrates. Proc R Soc Lond B 274:3097–3104
Clutton-Brock T, McAuliffe K (2009) Female mate choice in mammals. Q Rev Biol 84:3–27
Clutton-Brock TH, Guinness FE, Albon SD (1982) Red deer: behavior and ecology of two sexes. University of Chicago, Chicago
Clutton-Brock TH, Albon SD, Guiness FD (1988) Reproductive success in male and female red deer. In: Clutton-Brock T (ed) Reproductive success. University of Chicago Press, Chicago, pp 325–343
Coltman DW, Smith JA, Bancroft DR, Pilkington J, MacColl ADC, Clutton-Brock TH, Pemberton JM (1999) Density-dependent variation in lifetime breeding success and natural and sexual selection in Soay rams. Am Nat 154:730–746
Crockett C, Janson C (2000) Infanticide in red howlers: female group size, male membership, and a possible link to folivory. In: van Schaik C, Janson C (eds) Infanticide by males and its implications. Cambridge University Press, Cambridge, pp 75–98
Crow JF (1958) Some possibilities for measuring selection intensities in man. Hum Biol 30:1–13
Darwin C (1871) The descent of man and selection in relation to sex. Princeton University Press, Princeton
Downhower JF, Blumer LS, Brown L (1987) Opportunity for selection—an appropriate measure for evaluating variation in the potential for selection. Evolution 41:1395–1400
Dunbar R (1984) Reproductive decisions: an economic analysis of Gelada baboon social strategies. Princeton University Press, Princeton
Elgar MA (1989) Predator vigilance and group-size in mammals and birds—a critical review of the empirical evidence. Biol Rev Camb Phil Soc 64:13–33
Fashing PJ (2001) Activity and ranging patterns of guerezas in the Kakamega Forest: intergroup variation and implications for intragroup feeding competition. Int J Primatol 22:549–577
Gatti S, Levrero F, Menard N, Gautier-Hion A (2004) Population and group structure of western lowland gorillas (Gorilla gorilla gorilla) at Lokoué, Republic of Congo. Am J Primatol 63:111–123
Gerald CN (1995) Demography of the Virunga mountain gorilla (Gorilla gorilla beringei). MSc thesis, Princeton University, Princeton
Gillespie TR, Chapman CA (2001) Determinants of group size in the red colobus monkey (Procolobus badius): an evaluation of the generality of the ecological-constraints model. Behav Ecol Sociobiol 50:329–338
Hamilton WD (1971) Geometry for the selfish herd. J Theor Biol 31:294–311
Harcourt AH, Greenberg J (2001) Do gorilla females join males to avoid infanticide? A quantitative model. Anim Behav 62:905–915
Harcourt AH, Stewart KJ (2007) Gorilla society: conflict, compromise, and cooperation between the sexes. University of Chicago Press, Chicago
Harcourt AH, Stewart KS, Fossey D (1976) Male emigration and female transfer in wild mountain gorilla. Nature 263:226–227
Harris TR (2006) Between-group contest competition for food in a highly folivorous population of black and white colobus monkeys (Colobus guereza). Behav Ecol Sociobiol 61:317–329
Hodge SJ, Manica A, Flower TP, Clutton-Brock TH (2008) Determinants of reproductive success in dominant female meerkats. J Anim Ecol 77:92–102
Hrdy SB (1974) Male-male competition and infanticide among the langurs (Presbytis entellus) of Abu, Rajasthan. Folia Primatol 22:19–58
Isbell LA (1991) Contest and scramble competition: patterns of female aggression and ranging behavior in primates. Behav Ecol 2:143–155
Janson CH, Goldsmith ML (1995) Predicting group size in primates—foraging costs and predation risks. Behav Ecol 6:326–336
Jeffery KJ, Abernethy KA, Tutin CEG, Anthony NA, Bruford MW (2007) Who killed Porthos? Genetic tracking of a gorilla death. Integr Zool 2:111–119
Jones AG (2009) On the opportunity for sexual selection, the Bateman gradient and the maximum intensity of sexual selection. Evolution 63:1673–1684
Jones AG, Ratterman NL (2009) Mate choice and sexual selection: what have we learned since Darwin? Proc Natl Acad Sci U S A 106:10001–10008
Kalpers J, Williamson EA, Robbins MM, McNeilage A, Nzamurambaho A, Lola N, Mugiri G (2003) Gorillas in the crossfire: population dynamics of the Virunga mountain gorillas over the past three decades. Oryx 37:326–337
Kappeler PM (1997) Intrasexual selection and testis size in strepsirhine primates. Behav Ecol 8:10–19
Kelly CD (2008) The interrelationships between resource-holding potential, resource-value and reproductive success in territorial males: how much variation can we explain? Behav Ecol Sociobiol 62:855–871
Lande R (1981) Models of speciation by sexual selection on polygenic traits. Proc Natl Acad Sci U S A 78:3721–3725
Lawler RR (2007) Fitness and extra-group reproduction in male Verreaux’s sifaka: an analysis of reproductive success from 1989–1999. Am J Phys Anthropol 132:267–277
Le Boeuf BJ, Reiter J (1988) Lifetime reproductive success in northern elephant seals. In: Clutton-Brock T (ed) Reproductive success. University of Chicago Press, Chicago, pp 344–362
Lee AM, Engen S, Saether BE (2008) Understanding mating systems: a mathematical model of the pair formation process. Theor Popul Biol 73:112–124
Leigh SR, Shea BT (1995) Ontogeny and the evolution of adult body-size dimorphism in apes. Am J Primatol 36:37–60
Levrero F (2005) Structure d’une population de gorilles (Gorilla g. gorilla) visitant une clairière forestière—nature et rôle des rencontres intergroupes dans sa dynamique. PhD thesis, University of Rennes, Rennes
Lindenfors P (2002) Sexually antagonistic selection on primate size. J Evol Biol 15:595–607
Lindenfors P, Tullberg BS, Biuw M (2002) Phylogenetic analyses of sexual selection and sexual size dimorphism in pinnipeds. Behav Ecol Sociobiol 52:188–193
McElligott AG, Hayden TJ (2000) Lifetime mating success, sexual selection and life history of fallow bucks (Dama dama). Behav Ecol Sociobiol 48:203–210
McElligott AG, Altwegg R, Hayden TJ (2002) Age-specific survival and reproductive probabilities: evidence for senescence in male fallow deer (Dama dama). Proc R Soc Lond B 269:1129–1137
Mills SC, Grapputo A, Koskela E, Mappes T (2007) Quantitative measure of sexual selection with respect to the operational sex ratio: a comparison of selection indices. Proc R Soc Lond B 274:143–150
Mitani JC, GrosLouis J, Manson JH (1996) Number of males in primate groups: comparative tests of competing hypotheses. Am J Primatol 38:315–332
Moller AP, Alatalo RV (1999) Good-genes effects in sexual selection. Proc R Soc Lond B 266:85–91
Mundry R, Fischer J (1998) Use of statistical programs for nonparametric tests of small samples often leads to incorrect P values: examples from Animal Behaviour. Anim Behav 56:256–259
Nowell AA (2005) Behavioural development in wild western lowland gorillas (Gorilla gorilla gorilla). PhD thesis, University of Liverpool, Liverpool
Nowell AA, Fletcher AW (2007) Development of independence from the mother in Gorilla gorilla gorilla. Int J Primatol 28:441–455
Oufiero CE, Garland T (2007) Evaluating performance costs of sexually selected traits. Funct Ecol 21:676–689
Packer C, Herbst L, Pusey AE, Bygott JD, Hanby JP, Cairns SJ, Borgerfoff-Muller M (1988) Reproductive success in lions. In: Clutton-Brock T (ed) Reproductive success. University of Chicago Press, Chicago, pp 363–383
Packer C, Pusey A, Eberly L (2001) Egalitarianism in female African lions. Science 293:690–693
Parnell R (2002a) The social structure and behaviour of western lowland gorillas (Gorilla gorilla gorilla) at Mbeli Bai, Republic of Congo. PhD thesis, University of Stirling, Stirling
Parnell RJ (2002b) Group size and structure in western lowland gorillas (Gorilla gorilla gorilla) at Mbeli Bai, Republic of Congo. Am J Primatol 56:193–206
Pereira ME, Clutton-Brock TH, Kappeler PM (2000) Understanding male primates. In: Kappeler PM (ed) Primate males. Cambridge University Press, Cambridge, pp 271–277
Pradhan GR, van Schaik C (2008) Infanticide-driven intersexual conflict over matings in primates and its effects on social organization. Behaviour 145:251–275
Pradhan GR, Van Schaik CP (2009) Why do females find ornaments attractive? The coercion-avoidance hypothesis. Biol J Linn Soc 96:372–382
Robbins MM (2003) Behavioral aspects of sexual selection in mountain gorillas. In: Jones CB (ed) Sexual selection and reproductive competition in primates: new perspectives and directions. American Society of Primatologists, Norman, pp 477–501
Robbins MM (2009) Male aggression toward females in mountain gorillas: courtship or coercion? In: Muller MN, Wrangham R (eds) Sexual coercion in primates and humans: an evolutionary perspective on male aggression against females. Harvard University Press, Cambridge
Robbins AM, Robbins MM (2005) Fitness consequences of dispersal decisions for male mountain gorillas (Gorilla beringei beringei). Behav Ecol Sociobiol 58:295–309
Robbins MM, Sawyer SC (2007) Intergroup encounters in mountain gorillas of Bwindi Impenetrable National Park, Uganda. Behaviour 144:1497–1519
Robbins MM, Bermejo M, Cipolletta C, Magliocca F, Parnell RJ, Stokes E (2004) Social structure and life-history patterns in western gorillas (Gorilla gorilla gorilla). Am J Primatol 64:145–159
Robbins MM, Robbins AM, Gerald-Steklis N, Steklis HD (2007) Socioecological influences on the reproductive success of female mountain gorillas (Gorilla beringei beringei). Behav Ecol Sociobiol 61:919–931
Robbins AM, Stoinski TS, Fawcett KA, Robbins MM (2009a) Does dispersal cause reproductive delays in female mountain gorillas? Behaviour 146:525–549
Robbins AM, Stoinski TS, Fawcett KA, Robbins MM (2009b) Socioecological influences on the dispersal of female mountain gorillas-evidence of a second folivore paradox. Behav Ecol Sociobiol 63:477–489
Robinson MR, Pilkington JG, Clutton-Brock TH, Pemberton JM, Kruuk LEB (2006) Live fast, die young: trade-offs between fitness components and sexually antagonistic selection on weaponry in Soay sheep. Evolution 60:2168–2181
Setchell JM, Charpentier M, Wickings EJ (2005) Sexual selection and reproductive careers in mandrills (Mandrillus sphinx). Behav Ecol Sociobiol 58:474–485
Shuster SM, Wade MJ (2003) Mating systems and strategies. Princeton University Press, Princeton
Sicotte P (1993) Intergroup encounters and female transfer in mountain gorillas—influence of group composition on male behavior. Am J Primatol 30:21–36
Smith RJ, Jungers WL (1997) Body mass in comparative primatology. J Hum Evol 32:131–177
Spong GF, Hodge SJ, Young AJ, Clutton-Brock TH (2008) Factors affecting the reproductive success of dominant male meerkats. Mol Ecol 17:2287–2299
Steenbeek R, van Schaik CP (2001) Competition and group size in Thomas’s langurs (Presbytis thomasi): the folivore paradox revisited. Behav Ecol Sociobiol 49:100–110
Steenbeek R, Sterck EHM, DeVries H, van Hooff JARAM (2000) Costs and benefits of the one-male, age-graded and all-male phase in wild Thomas’s langur groups. In: Kappeler PM (ed) Primate males. Cambridge University Press, Cambridge, pp 130–145
Sterck EHM, Watts DP, van Schaik CP (1997) The evolution of female social relationships in nonhuman primates. Behav Ecol Sociobiol 41:291–309
Stoinski TS, Vecellio V, Ngaboyamahina T, Ndagijimana F, Rosenbaum S, Fawcett KA (2009) Proximate factors influencing dispersal decisions in male mountain gorillas, Gorilla beringei beringei. Anim Behav 77:1155–1164
Stokes EJ (2004) Within-group social relationships among females and adult males in wild western lowland gorillas (Gorilla gorilla gorilla). Am J Primatol 64:233–246
Stokes EJ, Parnell RJ, Olejniczak C (2003) Female dispersal and reproductive success in wild western lowland gorillas (Gorilla gorilla gorilla). Behav Ecol Sociobiol 54:329–339
Strassmann BI, Gillespie B (2003) How to measure reproductive success. Am J Human Biol 15:361–369
Sutherland WJ (1985) Chance can produce a sex difference in variance in mating success and explain Bateman’s data. Anim Behav 33:1349–1352
Thoren S, Lindenfors P, Kappeler PM (2006) Phylogenetic analyses of dimorphism in primates: evidence for stronger selection on canine size than on body size. Am J Phys Anthropol 130:50–59
Trail PW (1985) The intensity of selection—intersexual and interspecific comparisons require consistent measures. Am Nat 126:434–439
van Schaik CP (1983) Why are diurnal primates living in groups. Behaviour 87:120–144
van Schaik CP (1989) The ecology of social relationships amongst female primates. In: Standon V, Foley RA (eds) Comparative socioecology: the behavioural ecology of humans and other mammals. Blackwell Scientific Publications, Oxford, pp 195–218
van Schaik C, Janson C (2000) Infanticide by males and its implications. Cambridge University Press, Cambridge
van Schaik CP, Kappeler PM (1997) Infanticide risk and the evolution of male-female association in primates. Proc R Soc Lond B 264:1687–1694
van Schaik CP, Pradhan GR, van Noordwijk MA (2004) Infanticide by males, sex, and harassment in primates. In: Kappeler PM, van Schaik CP (eds) Sexual selection in primates, new and comparative perspectives. Cambridge University Press, Cambridge, pp 141–163
Vanpe C, Kjellander P, Galan M, Cosson JF, Aulagnier S, Liberg O, Hewison AJM (2008) Mating system, sexual dimorphism, and the opportunity for sexual selection in a territorial ungulate. Behav Ecol 19:309–316
Wade MJ (1979) Sexual selection and variance in reproductive success. Am Nat 114:742–747
Wade MJ (1987) Measuring sexual selection. In: Bradbury JW, Andersson MB (eds) Sexual selection: testing the alternatives. Chichester, New York, pp 197–207
Wade MJ, Arnold SJ (1980) The intensity of sexual selection in relation to male sexual-behavior, female choice, and sperm precedence. Anim Behav 28:446–461
Wade MJ, Shuster SM (2004) Sexual selection: harem size and the variance in male reproductive success. Am Nat 164:E83–E89
Watts DP (1989) Infanticide in mountain gorillas—new cases and a reconsideration of the evidence. Ethology 81:1–18
Watts DP (1990) Ecology of gorillas and its relation to female transfer in mountain gorillas. Int J Primatol 11:21–45
Watts DP (1991) Mountain gorilla reproduction and sexual behavior. Am J Primatol 24:211–225
Watts DP (2000) Causes and consequences of variation in male mountain gorilla life histories and group membership. In: Kappeler PM (ed) Primate males. Cambridge University Press, Cambridge, pp 169–180
West PM, Packer C (2002) Sexual selection, temperature, and the lion’s mane. Science 297:1339–1343
Williams JM, Oehlert GW, Carlis JV, Pusey AE (2004) Why do male chimpanzees defend a group range? Anim Behav 68:523–532
Yamagiwa J, Kahekwa J (2001) Dispersal patterns, group structure, and reproductive parameters of eastern lowland gorillas at Kahuzi in the absence of infanticide. In: Robbins MM, Sicotte P, Stewart K (eds) Mountain gorillas: three decades of research at Karisoke. Cambridge University Press, Cambridge, pp 89–122
Zahavi A (1975) Mate selection—selection for a handicap. J Theor Biol 53:205–214
Acknowledgements
We thank the Ministère de l’Économie Forestière for permission to work in the Nouabalé-Ndoki National Park and Wildlife Conservation Society’s Congo Program for crucial logistical and administrative support. Special thanks are due to numerous research assistants that contributed to the demographic data at Mbeli Bai. Financial support to the Mbeli Bai Study is provided by Brevard Zoo, The Columbus Zoo and Aquarium, Cincinnati Zoo and Botanical Garden, Cleveland Metropark Zoo, Disney Worldwide Conservation Fund, Margot Marsh Biodiversity Fund, Houston Zoo, Jacksonville Zoo, Knoxville Zoo, Little Rock Zoo, National Geographic Society, Santa Barbara Zoo, Sea World & Busch Gardens Conservation Fund, Toronto Zoo, US Fish and Wildlife Service, Wildlife Conservation Society, and Woodland Park Zoo. This study was also supported by the German Academic Exchange Service (DAAD), the Leakey Foundation, and the Max Planck Society.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Kappeler
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Breuer, T., Robbins, A.M., Olejniczak, C. et al. Variance in the male reproductive success of western gorillas: acquiring females is just the beginning. Behav Ecol Sociobiol 64, 515–528 (2010). https://doi.org/10.1007/s00265-009-0867-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-009-0867-6