Abstract
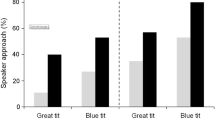
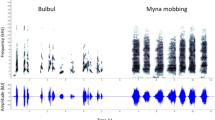
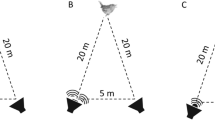
Migrating animals face numerous mortality risks, such as novel predators with which they may not be accustomed. Most animals can recognize predators innately; however, additional predator information can be collected to enhance familiarity. Because migrating birds rarely participate in mobs, they may seek alternative information sources such as cues provided by other birds that can provide information on predator location, identity, and degree of threat. We predicted that Nearctic–Neotropical migrants (hereafter, “migrants”) would react to vocal antipredator cues (e.g., mob-calls) of species residing in areas through which they migrate. To test this, we conducted experiments in Belize during spring migration, using playbacks of mob-calls of black-capped chickadees (Poecile atricapillus) and blue-gray tanagers (Thraupis episcopus); tanagers are familiar to all birds in Belize; chickadees are novel to residents but familiar to migrants. This also allowed us to assess response to novel and out-of-context antipredator signals. Resident birds did not respond to novel chickadee mob-calls, but did respond to familiar tanager calls. Birds overwintering south of our study area, which were migrating during our study, responded most strongly to chickadee playbacks. Conversely, individuals of species that include our study area in their winter range did not respond to either playback. This is the first evidence that birds react to vocal antipredator cues during migration, which may be a strategy used by migrants to learn about predators. Although residents failed to recognize a foreign cue, migrating birds responded most strongly to the out-of-context chickadee cue, associated with breeding grounds >2,000 km northward.


Similar content being viewed by others
References
Belisle M, Desrochers A (2002) Gap-crossing decisions by forest birds: an empirical basis for parameterizing spatially-explicit, individual-based models. Landsc Ecol 17:219–231
Betts MG, Hadley AS, Doran PJ (2005) Avian mobbing response is restricted by territory boundaries: experimental evidence from two species of forest warblers. Ethology 111:821–835
Boback SM (2005) Natural history and conservation of island boas (Boa constrictor) in Belize. Copeia 4:880–885
Brawn JD, Collins TM, Medina M, Bermingham E (1996) Associations between physical isolation and geographical variation within three species of Neotropical birds. Mol Ecol 5:33–46
Brown GE (2003) Learning about danger: chemical alarm cues and local risk assessment in prey fishes. Fish Fish 4:227–234
Caro TM (2005) Antipredator defenses in birds and mammals. University of Chicago Press, Chicago
Chinchilla FA (1997) Diets of Panthera onca, Felis concolor and Felis pardalis (Carnivora: Felidae) in Parque Nacional Corcovado, Costa Rica. Rev Biol Trop 45:1223–1229
Chivers DP, Smith RJF (1994) Fathead minnows, Pimephales promelas, acquire predator recognition when alarm substance is associated with the sight of unfamiliar fish. Anim Behav 48:597–605
Conover MR (1987) Acquisition of predator information by active and passive mobbers in ring-billed gull colonies. Behaviour 102:41–57
Curio E (1978) The adaptive significance of mobbing. I. Teleonomic hypotheses and predictions. Z Tierpsychol 48:175–183
Danchin E, Giraldeau LA, Valone TJ, Wagner RH (2004) Public information: from nosy neighbors to cultural evolution. Science 305:487–491
DaSilva JMC, Uhl C, Murray G (1996) Plant succession, landscape management, and the ecology of frugivorous birds in abandoned Amazonian pastures. Conserv Biol 10:491–503
Deecke VB, Slater PJB, Ford JKB (2002) Selective habituation shapes acoustic predator recognition in harbour seals. Nature 420:171–173
Dierschke V, Delingat J (2001) Stopover behaviour and departure decision of northern wheatears, Oenanthe oenanthe, facing different onward non-stop flight distances. Behav Ecol Sociobiol 50:535–545
Dolby AS, Grubb TC (1998) Benefits to satellite members in mixed-species foraging groups: an experimental analysis. Anim Behav 56:501–509
Drent R, Both C, Green M, Madsen J, Piersma T (2003) Pay-offs and penalties of competing migratory schedules. Oikos 103:274–292
Dugatkin LA, Godin JGJ (1992) Prey approaching predators: a cost–benefit perspective. Ann Zool Fenn 29:233–252
Edelaar P, Wright J (2006) Potential prey make excellent ornithologists: adaptive, flexible responses towards avian predation threat by Arabian Babblers Turdoides squamiceps living at a migratory hotspot. Ibis 148:664–671
Ficken MS, Ficken RW, Witkin SR (1978) Vocal repertoire of the black-capped Chickadee. Auk 95:34–48
Fitzgibbon CD (1994) The costs and benefits of predator inspection behavior in Thomson gazelles. Behav Ecol Sociobiol 34:139–148
Godin JGJ, Davis SA (1995) Who dares, benefits—predator approach behavior in the guppy (Poecilia reticulata) deters predator pursuit. Proc R Soc Lond B 259:193–200
Halupka K, Halupka L (1997) The influence of reproductive season stage on nest defence by meadow pipits (Anthus pratensis). Ethol Ecol Evol 9:89–98
Hames RS, Lowe JD, Barker Swarthout S, Rosenberg KV (2006) Understanding the risk to neotropical migrant bird species of multiple human-caused stressors: elucidating processes behind the patterns. Ecol Soc 11, article 24 [online] URL: http://www.ecologyandsociety.org/vol11/iss1/art24/
Hilty S (1994) Birds of tropical America. University of Texas Press, Austin
Hurd CR (1996) Interspecific attraction to the mobbing calls of black-capped chickadees (Parus atricapillus). Behav Ecol Sociobiol 38:287–292
Ishihara M (1987) Effect of mobbing toward predators by the damselfish Pomacentrus coelestis (Pisces, Pomacentridae). J Ethol 5:43–52
Jones HL (2003) Birds of Belize. University of Texas Press, Austin
Johnson FR, McNaughton EJ, Shelley CD, Blumstein DT (2003) Mechanisms of heterospecific recognition in avian mobbing calls. Aust J Zool 51:577–585
Krams I, Krama T (2002) Interspecific reciprocity explains mobbing behaviour of the breeding chaffinches, Fringilla coelebs. Proc R Soc Lond B 269:2345–2350
Langham GM, Contreras TA, Sieving KE (2006) Why pishing works: Titmouse (Paridae) scolds elicit a generalized response in bird communities. Ecoscience 13:485–496
Latta SC, Wunderle JM (1996) The composition and foraging ecology of mixed-species flocks in pine forests of Hispaniola. Condor 98:595–607
Leal M, Rodriguez-Robles JA (1997) Signalling displays during predator–prey interactions in a Puerto Rican anole, Anolis cristatellus. Anim Behav 54:1147–1154
Lind J, Cresswell W (2005) Determining the fitness consequences of antipredation behavior. Behav Ecol 16:945–956
Lind J, Cresswell W (2006) Anti-predation behaviour during bird migration; the benefit of studying multiple behavioural dimensions. J Ornithol 147:310–316
Lind J, Jöngren F, Nilsson J, Alm DS, Strandmark A (2005) Information, predation risk and foraging decisions during mobbing in Great Tits Parus major. Ornis Fenn 82:89–96
Losito MP, Mirarchi RE, Baldassarre GA (1989) New techniques for time–activity studies of avian flocks in view-restricted habitats. J Field Ornithol 60:388–396
Manomet Observatory for Conservation Sciences (1996) Impacts of silvicultural trials on birds and tree regeneration in the Chiquibul Forest Reserve, Belize. Supplement: birds banded and saplings tagged. Report to Ministry of Natural Resources, Belmopan, Belize
Marler P (1957) Specific distinctiveness in the communication signals of birds. Behaviour 11:13–39
Mathis A, Chivers DP, Smith RJF (1996) Cultural transmission of predator recognition in fishes: intraspecific and interspecific learning. Anim Behav 51:185–201
McLean IG, Rhodes G (1991) Enemy recognition and response in birds. Curr Ornithol 8:173–211
Mettke-Hofmann C, Gwinner E (2004) Differential assessment of environmental information in a migratory and a nonmigratory passerine. Anim Behav 68:1079–1086
Owings DH, Coss RG (1977) Snake mobbing by California ground squirrels—adaptive variation and ontogeny. Behaviour 62:50–69
Pijanowska J (1997) Alarm signals in Daphnia? Oecologia 112:12–16
Pyle P (1997) Identification guide to North American birds, part 1. Slate Creek, Bolinas
R Development Core Team (2006) R: a language environment for statistical computing. R Foundation for Statistical Computing, Vienna
Shedd DH (1982) Seasonal variation and function of mobbing and related antipredator behaviors of the American Robin (Turdus migratorius). Auk 99:342–346
Sieving KE, Contreras TA, Maute KL (2004) Heterospecific facilitation of forest-boundary crossing by mobbing understory birds in North–Central Florida. Auk 121:738–751
Slusarczyk M, Rygielska E (2004) Fish faeces as the primary source of chemical cues inducing fish avoidance diapause in Daphnia magna. Hydrobiologia 526:231–234
Smith SM (1993) Black-capped chickadee (Parus atricapillus). In: Poole A, Gill F (eds) The birds of North America, no 39. The Birds of North America, Philadelphia
Srivastava A (1991) Cultural transmission of snake-mobbing in free-ranging Hanuman langurs. Folia Primatol 56:117–120
Stutchbury BJM, Morton ES (2001) Behavioral ecology of tropical birds. Academic, London
Templeton CN, Greene E (2007) Nuthatches eavesdrop on variations in heterospecific chickadee mobbing alarm calls. Proc Nat Acad Sci 104:5479–5482
Templeton CN, Greene E, Davis K (2005) Allometry of alarm calls: black-capped chickadees encode information about predator size. Science 308:1934–1937
Tikkanen P, Muotka T, Huhta A (1996) Fishless-stream mayflies express behavioural flexibility in response to predatory fish. Anim Behav 51:1391–1399
Turcotte Y, Desrochers A (2002) Playbacks of mobbing calls of black-capped chickadees help estimate the abundance of forest birds in winter. J Field Ornithol 73:303–307
Veen T, Richardson DS, Blaakmeer K, Komdeur J (2000) Experimental evidence for innate predator recognition in the Seychelles warbler. Proc R Soc Lond B 267:2253–2258
Winn HE (1960) Biology of the brook stickleback Eucalia inconstans (Kirtland). Am Midl Nat 63:424–438
Acknowledgment
We are indebted to Trina Fitzgerald (of the Atlantic Bird Observatory) for orchestrating the independent bird-banding studies and commenting on drafts of this manuscript. Two anonymous referees also provided useful comments. Our appreciation is also extended to those who assisted with fieldwork: Kate Dalley, Trina Fitzgerald, Jason Glode, Pete Goulet, and Tina Leonard. We also thank Isidro and Calistro Bol for their field support and station management. Chris Templeton kindly provided recordings of black-capped chickadee calls, while tanager calls were obtained from www.naturesongs.com. The Natural Sciences and Engineering Research Council of Canada (postdoctoral fellowship to JJN, Discovery grants to PDT and LMR), Queen’s University, the National Geographic Society, and the Percy Sladen Memorial Fund supported our research. All field methods used in this research complied with the current laws of Canada and Belize.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Bednekoff
Electronic supplementary material
Below is the link to the electronic supplementary material.
S1
Numbers and migrant status of all migratory bird species captured during passive mist-netting or detected at playbacks black-capped chickadee mob-calls, blue-gray tanager mob-calls, static, or no sound at all in Belize, Central America (DOC 67 KB)
S2
Numbers of all resident bird species captured during passive mist-netting or detected at playbacks black-capped chickadee mob-calls, blue-gray tanager mob-calls, static, or no sound at all in Belize, Central America (DOC 107 KB)
Rights and permissions
About this article
Cite this article
Nocera, J.J., Taylor, P.D. & Ratcliffe, L.M. Inspection of mob-calls as sources of predator information: response of migrant and resident birds in the Neotropics. Behav Ecol Sociobiol 62, 1769–1777 (2008). https://doi.org/10.1007/s00265-008-0605-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-008-0605-5