Abstract
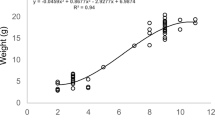
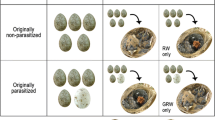
The quality and quantity of food delivered to young are among the major determinants of fitness. A parental provisioning capacity is known to increase with body size. Therefore, brood parasitism provides an opportunity to test the effects of varying provisioning abilities of different-sized hosts on parasitic chick growth and fledging success. Knowledge of growth patterns of common cuckoo, Cuculus canorus, chicks in nests of common hosts is very poor. Moreover, no study to date has focused on any currently unused hosts (i.e., suitable cuckoo host species in which parasitism is currently rare or absent). Here, I compare the growth performance of cuckoo chicks in nests of a common host (the reed warbler, Acrocephalus scirpaceus) and two unparasitized hosts (the song thrush, Turdus philomelos, and the blackbird, Turdus merula). Parasitic chicks were sole occupants of the observed nests, thus eliminating the confounding effect of competition with host chicks. Experiments revealed striking differences in parasitic chick growth in the two closely related Turdus hosts. Cuckoo chicks cross-fostered to song thrush nests grew much quicker and attained much higher mass at fledging than those in nests of their common reed warbler host. Alternatively, parasitic chicks in blackbird nests grew poorly and did not survive until fledging. I discuss these observations with respect to host selection by parasitic cuckoos.



Similar content being viewed by others
References
Alvarez F (1994) Rates of weight increase of cuckoo (Cuculus canorus) and host (Cercotrichas galactotes) chicks. Ardeola 41:63–65
Brooke ML, Davies NB (1989) Provisioning of nestling cuckoos Cuculus canorus by reed warbler Acrocephalus scirpaceus hosts. Ibis 131:250–256
Bussmann J (1947) Wachstum und Jugendzeit eines Kuckucks. Ornithol Beob 44:41–49
Cramp S (ed) (1988) The birds of the Western Palearctic. Tyrant flycatchers to thrushes, vol 5. Oxford University Press, Oxford
Cramp S (ed) (1992) The birds of the Western Palearctic. Warblers, vol 6. Oxford University Press, Oxford
Davies NB (1986) Reproductive success of dunnocks, Prunella modularis, in a variable mating system. 1. Factors influencing provisioning rate, nestling weight and fledgling success. J Anim Ecol 55:123–138
Davies NB (2000) Cuckoos, cowbirds and other cheats. T & AD Poyser, London
Davies NB, Brooke ML (1989) An experimental study of co-evolution between the cuckoo, Cuculus canorus, and its hosts. I. Host egg discrimination. J Anim Ecol 58:207–224
Glassey B, Forbes S (2003) Why brown-headed cowbirds do not influence red-winged blackbird parent behaviour. Anim Behav 65:1235–1246
Glue D, Morgan R (1972) Cuckoo hosts in British habitats. Bird Study 19:187–192
Grim T (1999) Food of great reed warbler (Acrocephalus arundinaceus) young. Sylvia 35:93–99 (in Czech, with summary in English)
Grim T (2005a) Host recognition of brood parasites: implications for methodology in studies of enemy recognition. Auk 122:530–543
Grim T (2005b) Mimicry vs. similarity: which resemblances between brood parasites and their hosts are mimetic and which are not? Biol J Linn Soc 84:69–78
Grim T (2006) The evolution of nestling discrimination by hosts of parasitic birds: why is rejection so rare? Evol Ecol Res 8:785–802
Grim T, Honza M (1997) Differences in parental care of reed warbler (Acrocephalus scirpaceus) to its own nestlings and parasitic cuckoo (Cuculus canorus) chicks. Folia Zool 46:135–142
Grim T, Honza M (2001a) Differences in behaviour of closely related thrushes (Turdus philomelos and T. merula) to experimental parasitism by the common cuckoo Cuculus canorus. Biologia 56:549–556
Grim T, Honza M (2001b) Does supernormal stimulus influence parental behaviour of the cuckoo’s host? Behav Ecol Sociobiol 49:322–329
Grim T, Kleven O, Mikulica O (2003) Nestling discrimination without recognition: a possible defence mechanism for hosts towards cuckoo parasitism? Proc R Soc Lond B 270:S73–S75
Honza M, Picman J, Grim T, Novak V, Capek M, Mrlik V (2001) How to hatch from an egg of great structural strength: a study of the common cuckoo. J Avian Biol 32:249–255
Khayutin SN, Dmitrieva LP, Tartygina NG, Aleksandrov LI (1982) The behaviour of a nestling of Cuculus canorus in the nest of Phoenicurus phoenicurus. Zool Z 61:1063–1077 (in Russian with English summary)
Kilner RM (2003) How selfish is a cowbird nestling? Anim Behav 66:569–576
Kilner RM, Davies NB (1999) How selfish is a cuckoo chick? Anim Behav 58:797–808
Kilner RM, Madden JR, Hauber ME (2004) Brood parasitic cowbird nestlings use host young to procure resources. Science 305:877–879
Kilpatrick AM (2002) Variation in growth of brown-headed cowbird (Molothrus ater) nestlings and energetic impacts on their host parents. Can J Zool 80:145–153
Kleven O, Moksnes A, Røskaft E, Honza M (1999) Host species affects the growth rate of cuckoo (Cuculus canorus) chicks. Behav Ecol Sociobiol 47:41–46
Lindén M, Gustafsson L, Pärt T (1992) Selection on fledging mass in the collared flycatcher and the great tit. Ecology 73:336–343
Magrath RD (1991) Nestling weight and juvenile survival in the blackbird Turdus merula. J Anim Ecol 60:335–351
Magrath RD (1992) Roles of egg mass and incubation pattern in establishment of hatching hierarchies in the blackbird (Turdus merula). Auk 109:474–487
Martin TE (1987) Food as a limit on breeding birds: a life-history perspective. Annu Rev Ecol Syst 18:453–487
Martin-Galvez D, Soler M, Soler JJ, Martin-Vivaldi M, Palomino JJ (2005) Food acquisition by common cuckoo chicks in rufous bush robin nests and the advantage of eviction behaviour. Anim Behav 70:1313–1321
Moksnes A, Røskaft E (1992) Responses of some rare cuckoo hosts to mimetic cuckoo eggs and foreign conspecific eggs. Ornis Scand 23:17–23
Moksnes A, Røskaft E (1995) Egg-morphs and host preference in the common cuckoo (Cuculus canorus): an analysis of cuckoo and host eggs from European museum collections. J Zool 236:625–648
Moksnes A, Røskaft E, Braa AT, Korsnes L, Lampe HM, Pedersen HC (1990) Behavioural responses of potential hosts towards artificial cuckoo eggs and dummies. Behaviour 116:64–89
Moskát C, Karcza Z, Csörgö T (2003) Egg rejection in European blackbirds (Turdus merula): the effect of mimicry. Ornis Fenn 80:86–91
Numerov AD (2003) Interspecific and intraspecific brood parasitism in birds. Federal State Unitary Enterprise Publish & Polygraf Corporation, Voronezh (in Russian, with English summary)
Rothstein SI, Robinson SK (1998) Parasitic birds and their hosts. Oxford University Press, New York
Saether B-E (1994) Food provisioning in relation to reproductive strategy in altricial birds: a comparison of two hypotheses. Evolution 48:1397–1406
SAS Institute (2000) SAS online document, version 8. SAS, Cary
Soler M (2001) Begging behaviour of nestlings and food delivery by parents: the importance of breeding strategy. Acta Ethol 4:59–63
Soler M (2002) Breeding strategy and begging intensity: influences on food delivery by parents and host selection by parasitic cuckoos. In: Wright J, Leonard ML (eds) The evolution of begging. Kluwer, Dordrecht, pp 413–427
Soler M, Soler JJ (1991) Growth and development of great spotted cuckoos and their magpie hosts. Condor 93:46–54
Starck JM, Ricklefs RE (eds) (1998) Avian growth and development. Evolution within the altricial–precocial spectrum. Oxford University Press, New York
Strausberger BM (1998) Temporal patterns of host availability, brown-headed cowbird brood parasitism, and parasite egg mass. Oecologia 116:267–274
Sutherland WJ, Newton I, Green RE (2004) Bird ecology and conservation. A handbook of techniques. Oxford University Press, Oxford
Trine CL (1998) Wood thrush population sinks and implications for the scale of regional conservation strategies. Conserv Biol 12:576–585
Werth I (1947) The growth of a young cuckoo. Br Birds 40:331–334
Wiley JW (1986) Growth of shiny cowbird and host chicks. Wilson Bull 98:126–131
Woolfenden BE, Gibbs HL, Sealy SG, McMaster DG (2003) Host use and fecundity of individual female brown-headed cowbirds. Anim Behav 66:95–106
Wyllie I (1981) The cuckoo. Batsford, London
Acknowledgements
Suggestions by three anonymous referees and the associate editor substantially improved this paper. I would like to thank M. E. Hauber, O. Kleven, E. Røskaft and V. Remeš for their comments on earlier versions of the MS. P. Procházka kindly provided some video recordings. I am grateful to all who helped with the fieldwork: A. Dvorská, M. Honza, O. Kleven, B. Matysioková, A. Moksnes, P. Procházka, E. Røskaft, G. Rudolfsen, and V. Šícha. When working on this paper, I was supported by grants from the Research Council of Norway, the Czech Ministry of Education (grants No. 153100012 and No. MSM6198959212), and the Grant Agency of the Czech Republic (206/03/D234). No nest was abandoned after the experiments were performed. The experiments were done under license from The Central Commission for Animal Welfare of the Czech Republic (No. 065/2002–V2) and in accordance with the laws and ethical guidelines of the Czech Republic.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Soler
Rights and permissions
About this article
Cite this article
Grim, T. Cuckoo growth performance in parasitized and unused hosts: not only host size matters. Behav Ecol Sociobiol 60, 716–723 (2006). https://doi.org/10.1007/s00265-006-0215-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-006-0215-z