Abstract
Genes of the major histocompatibility complex (MHC) are remarkably polymorphic. Several selection mechanisms have been invoked to account for this diversity, including disassortative mating preferences. In addition, eggs may discriminate between sperm based on MHC. To investigate the effects of MHC-genotype on fertilization success, we obtained mature gametes from ripe Arctic charr (Salvelinus alpinus) males and females captured on spawning grounds. The eggs of each female were divided into two batches, and by letting each of 2 males fertilize 1 of the batches, we obtained a total of 36 half-sibling batch-pairs. The semen was diluted to ensure that the two males in each half-sibling batch-pair contributed with the same number of sperm cells. We found that MHC-heterozygous males had significantly higher fertilization success than MHC-homozygous males and neither initial spermatocrit, sperm motility nor swimming velocity co-varied with difference in fertilization success. There was no effect of female genotype or female-male MHC-similarity on fertilization success. However, one MHC-allele was associated with increased fertilization success. It seems plausible that the difference in fertilization success between homo- and heterozygous males may be due to MHC-dependent sperm selection by the ovum.
Similar content being viewed by others
Introduction
In general, sexual selection is a result of males competing for access to female gametes (Eberhard 1996). Such competition can occur on many levels, including competition between individual males for copulations (Andersson 1994) and between ejaculates from different males for fertilization of the egg (Birkhead and Møller 1998). Furthermore, the female may influence the outcome of this competition by biasing preferred males over other males or some sperm over other sperm and thus potentially exert choice at any level, pre- or post-copulation (Andersson 1994; Eberhard, 1996). The egg itself may also exert choice between the sperm that reach it for fertilization (Eberhard 1996). Sperm selection by eggs has briefly been explored theoretically (Wedekind and Folstad 1994; Eberhard 1996), yet very few studies on the potential processes have been conducted (but see Rülicke et al. 1998 and Birkhead and Pizzari 2002). One example is the intriguing behavior of the comb jelly, Beroe ovata, egg pronucleus, which appears to investigate and choose amongst the different sperm that enter the egg (Carré and Sardet 1984). Also, in the beetle Callosobruchus maculatus, evidence for an effect of female genotype on the outcome of male sperm competition exists (Wilson et al. 1997). Yet, other studies have failed to document any evidence of post-copulatory choice (e.g. Stockley 1997).
A variety of potential benefits from mate and gamete choice have been suggested (Andersson 1994). In recent years, “good genes” models of sexual selection and, in particular, the Hamilton and Zuk (1982) hypothesis have attracted much attention and research on the immunological aspects of parasite-host co-evolutionary arms races (Hamilton and Poulin 1997). Genes of the major histocompatibility complex (MHC) are crucial in the specific immune response, as they code for the cell-membrane glycoproteins that present antigens to T-lymphocytes (e.g. Janeway and Travers 1997). Several selection mechanisms have been suggested to explain the unrivaled degree of polymorphism at the MHC-loci, but it has for various reasons proven difficult to verify direct pathogen-driven selection on MHC-genes (Apanius et al. 1997). Different MHC-alleles may present different antigens to T-cells and therefore confer varying degrees of resistance against different pathogens, setting the stage for frequency-dependent pathogen-driven selection. Furthermore, there might be a heterozygous advantage as MHC-gene expression is co-dominant, and heterozygotes could consequently be able to recognize a larger array of pathogens. Therefore, because of their large allelic variation and because this genetic variation results in individual variation in the ability to respond to immune challenge, the MHC-genes are excellent candidates for “compatible genes” (Tregenza and Wedell 2000) and “good genes” within parasite-mediated sexual selection (von Schantz et al. 1996; Jordan and Bruford 1998; Siva-Jothy and Skarstein 1998; Penn 2002). The MHC has been linked to mate preferences and sexual selection in several studies (Yamazaki et al. 1976; Egid and Brown 1989; Potts et al. 1991; Ober et al. 1994; Wedekind et al. 1995; von Schantz et al. 1996; Wedekind and Furi 1997; see Penn 2002 for a review).
In general, the teleost fish offers a good model system for the study of sperm selection by eggs. Fertilization is external, allowing in-vitro fertilizations to be conducted without much deviation from the natural fertilization process. Furthermore, the teleost egg itself has structures that can facilitate sperm discrimination. Teleost eggs are wrapped in an egg envelope (the chorion) in which a micropyle presents the only entrance for the sperm into the egg (Kamler 1992). When observed during in-vitro fertilization, spermatozoa can be seen moving along the surface of the chorion and entering the micropyle tract area, an indentation surrounding the micropyle in most teleosts, in a manner that suggests sperm guidance by a chemo-attraction system (Amanze and Iyengar 1990). The diameter of the teleost micropyle is usually just the width of a single sperm, and after a sperm enters the egg, the micropyle closes, which effectively blocks the entrance of other sperm. Therefore, during sperm attraction and passage through the micropyle-tract area, a potential exists for the egg to exert bias towards certain sperm over others. This bias could be influenced by MHC-dependent diploid or haploid influences on sperm phenotype.
The salmonid Arctic charr (Salvelinus alpinus) has additional life-history traits that make it well suited for the study of sperm-egg interactions. As salmonid eggs receive no parental care and are unusually large (Johnson 1980), handling and observation of the eggs after in-vitro fertilizations is straightforward and easy. Furthermore, females and dominant male Arctic charr experience sneak spawning by small, subdominant males (author’s personal observation). As the female is unable to completely prevent sneakers from spawning with her, the outcome of any female mate choice is confounded, emphasizing the importance of sperm selection by eggs. Finally, the presence of ejaculates from several males creates obvious potential for inter-ejaculate competition in addition to any intra-ejaculate competition that might be occurring.
In this study, we investigate whether male MHC-genotype correlates with fertilization success in the Arctic charr. By conducting split brood experiments, we investigate if heterozygous males fertilize more eggs than homozygous males. More specifically, we investigated if certain paternal alleles are associated with higher or lower fertilization success and if rare alleles are associated with higher fertilization success.
Methods
Sampling
Wild Arctic charr males and females, all ripe, were netted on spawning grounds in Lake Fjellfrøsvatn (Norway) during the 1995 spawning season, in late September/early October. Each individual was stripped for gametes by applying a gentle stroke from the anterior part of the abdomen towards the anal opening. Care was taken to ensure that the gametes were not activated by contact with water during stripping and subsequent storage.
On average, each female yielded 232 eggs (max.=298, min.=191) and each female’s eggs were randomly divided equally between 2 dry plastic containers (25 ml), which were then stored in darkness at 5°C to await fertilization. All eggs and milt samples were used within 5 h after stripping.
Fertilization procedures and sperm analysis
Prior to fertilization, we measured the spermatocrit (the percentage of the milt volume occupied by spermatozoa) of all milt samples by centrifuging a homogenized portion of the obtained milt in a capillary tube with a Compur mini centrifuge for 195 s at 11,500 rpm (repeatability, r2=0.83, P<0.001, n=16).
Each of the two egg batches from a female was fertilized by one male each and the two resulting half-sib groups constituted one split brood. Thus, the two half-sib groups of a split brood shared the mother but had different fathers. All sperm samples were used in order of collection, consequently minimizing the variation in storage time between the two samples of sperm used in each pair. Prior to fertilization, for each of the two fathers in the split brood, a mixture of 20 ml lake water and milt was made. The amount of milt added was adjusted according to its spermatocrit so that the two batches of eggs obtained from a female were always presented with the same amount of sperm (with a calculated spermatocrit of 0.01% in the total lake water/milt mixture), but from different males. This ensured that any difference in fertilization success between the two fathers of a split brood was independent of initial differences in sperm concentration (see below). Each male was involved in the fertilization of one split brood only, so that split broods from different females could be compared independent of the male effect on fertilization. Each milt/lake-water mixture was added to the assigned egg batch for fertilization within 10 s after mixing. This mixture was left for 2 min, after which the eggs were rinsed by replacing the water in the container twice. The containers were then filled with water and stored at 5°C awaiting transport to the hatchery. A total of 31 split broods were constructed, using eggs from 25 females (6 females had yielded enough eggs for 2 split broods) and sperm from 62 males. In the hatchery, broods were randomly allocated to individual compartments circulated by water without light except during short daily inspections. The experiment was terminated after 102 days, when all remaining eggs had become eye-ova, i.e. developed eyespots. Out of the eggs that never developed to this stage, 95% had developed readily identifiable visual signs of egg-white denaturation (unfertilized) within the first 2 days. We had no knowledge of any MHC-genotypes until after the fertilization experiment had ended.
Sperm samples from 28 males were analyzed further and percentage sperm motility (defined as percentage of sperm observed swimming during the observation period) and sperm swimming speed (defined as the distance covered during the observation period) were obtained using video footage of the sperm samples at 40*10 magnification. Sperm movements were quantified by tracking individual sperm (on average 26, SD=±13.03, sperm for each male) frame by frame on a monitor for 1 s between the 10th and 11th s after activation, i.e., the observation period. This procedure was conducted twice for each individual, and repeatability was rspeed2=0.48, n=11, P=0.02 and rmotility2=0.42, n=10, P<0.05. The average of the two repeated measures were used in the hypothesis testing.
Genotyping
Using PCR and denaturing gradient gel electrophoresis (DGGE), genotype of exon 2 from one MHC class II-B locus was determined for all the males. The amplified fragment spans the whole of the 2nd exon, coding for the B1 domain of the peptide-binding region of the class II MHC protein. The forward primer (5′-TAAACAGACAAAACAATGA-3′) was located in intron 1, 25 bp upstream of exon 2 and the reverse primer (3′-CACCTGTCTTGTCCAGTATGGCGGCCGCCCGTCCCGCCGCCCCCGCCCCGCCGCGGCCGC-5′) at the end of exon 2 and designed with a 3′ CG clamp to improve resolution on the DGGE. Genomic DNA was extracted and purified according to Laird et al. (1991) from small (approximately 20 mg) caudal fin cuts. Standard 25-µl PCR reactions were performed on a PE 9600 instrument (Perkin Elmer). The reaction contained 100 ng of template DNA, 5–6 pmol of each primer and dNTP (Pharmacia), MgCl2, reaction buffer and enzyme (AmpliTaq, Perkin Elmer) according to the supplier’s recommendations. Conditions for the PCR was: denaturation at 94°C for 30 s, 56°C, 30 s annealing and 72°C, 30 s extension repeated for 30–35 cycles. Each amplification was preceded by a 4-min denaturation at 94°C and a 10-min final extension at 72°C. All reactions included one negative control without template DNA.
To determine MHC genotypes we used DGGE, which after optimalization allowed separation of different exon 2 fragments of the same length based on sequence differences (Fisher and Lerman 1983; Myers et al. 1987). The combination of PCR and DGGE has previously been used to study MHC variation in Arctic charr and other salmonid fish and provides an easy and reliable method for MHC typing in new and less well-studied species compared to serological typing or restriction fragment length polymorphism, RFLP (Miller and Withler 1997; Olsén et al. 1998; Langefors et al. 2000).
DGGE was performed using 5%, 19:1 acrylamide/bisacrylamide, polyacrylamide parallel gels containing 1×TAE buffer and a gradient of urea and formamide. Denaturant concentrations in the gels ranged from 20% to 50% (Myers et al. 1987). Electrophoreses were performed in 1×TAE buffer heated to 60°C in electrophoreses cells and a buffer tank (C.B.S. Scientific, USA). For each electrophoresis, a volume of 10–15 μl PCR products per lane was run between 5 h and 6 h at 180 V. All gels contained three reference lanes with a standard sample containing alleles 1–5. The gels were stained with ethidium bromide (0.2 μg/ml 1×TAE) for 15 min and the gels were scanned with a fluoroimager (Amersham, USA). Bands on the gels were scored manually with the aid of a computer genotyping program (Amersham, USA). Bands separated by at least 0.5 mm on the gel were considered as separate bands. Most gels also showed heteroduplex bands formed in the PCR when DNA strands originating from different alleles in heterozygous samples reanneal and form a mismatched DNA fragment. Both homo- and heteroduplex bands were scored, which particularly increased the separation of heterozygous and homozygous individuals. We recovered the homoduplex bands of two to four different samples from each of the eight alleles from DGGE gels and used DNA sequencing to confirm the reliability of the DGGE based genotyping.
An MHC similarity index was calculated by counting the number of alleles shared between a male and the female whose eggs he fertilized. A male was assigned a score of 0 if he shared no alleles with the female, a score of 1 if they shared one allele, and 2 if they shared two alleles. Thus, a male that achieved a high MHC similarity index shared more MHC alleles with the female in the brood compared to a male with a low MHC similarity index.
The population frequencies of the two alleles in a male’s genotype were averaged to yield an index of allelic rareness (hereafter RI).
Statistical analyses were conducted using GenePop version 1.3 (Raymond and Rousset 1995), StatView 4.5 and JMP 5 for the Mac OS. All variables in the study had frequency distributions deviating from the normal distribution. We therefore conducted all statistical analyses using non-parametric tests. Wilcoxon signed rank tests were used to test for differences between paired half-sib groups, while Mann-Whitney U-tests were used to test for differences within split broods. Kendall rank correlations were used to test for associations between continuous variables.
Differences in fertilization success within split broods were compared across split broods using a measure of relative difference in fertilization success, which was calculated as the difference in fertilization success between the paired fathers of a split brood divided by the average fertilization success in the split brood.
Results
In the sample of 83 fish included in this study, 8 different MHC-alleles were found. This is a rather low number compared to MHC class II variation in many other species (Apanius et al. 1997), but a similar level of variation has been found in other salmonids and may be the effect of a bottleneck when populations formed after the last glaciation (Miller and Withler 1996). The allele distribution was nevertheless fairly uniform (Table 1), with the five most common alleles in frequencies ranging between 10 and 26%. The observed numbers of homozygous and heterozygous fish in the sample were compared to the Hardy Weinberg expectation. which revealed significantly more homozygous individuals in the sample than expected from random mating (Homozygous: exp. 14.9, obs. 20, Heterozygous: exp. 68, obs. 63, P=0.024; SE=0.0054).
The initial spermatocrit difference between paired fathers did not correlate with relative differences in fertilization success within the split brood (Kendall rank correlation: tau=0.20, Z=1.57, P=0.12, n=31). This suggests that initial differences in sperm-cell concentration were adequately controlled for by the sperm dilution procedure described above.
Of the 31 split broods initially constructed, 12 split broods had both an MHC-heterozygous father and an MHC-homozygous father (Table 2). That is, we obtained 12 independent comparisons of the differences in fertilization success between homo- and heterozygous males.
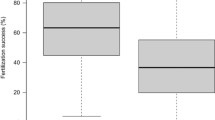
The unsigned relative difference in fertilization success was higher in split broods that had both a homo- and heterozygous father, compared to split broods where both fathers were heterozygous (Mann-Whitney: Z=−2.11, P=0.035, nhomo/hetero=12, nhetero/hetero=19) (Fig. 1). In no split brood were both fathers homozygous. Furthermore, looking within each brood, the heterozygous male had a significantly higher fertilization success than the homozygous male in 10 of the 12 split broods (Wilcoxon signed rank: Z=−2.51, P=0.01, n=12 pairs) (Fig. 2). There was no significant difference between the paired MHC-homozygous and -heterozygous males in initial spermatocrit (Wilcoxon signed rank: Z=−0.62, P=0.53, n=12 pairs). Sperm motility and swimming speed did not co-vary with male MHC-heterozygosity either (Wilcoxon signed rank: motility: Z=−1.67, P=0.61, nhomo=6, nhetero=23; velocity: Z=−0.75, P=0.45, nhomo=6, nhetero=23). The statistical power of the Wilcoxon signed rank tests of differences in the various sperm-quality estimates between homo- and heterozygous males were calculated, producing power values ranging from 0.51 to 0.73.
Unsigned mean (±1 SE) relative difference in fertilization success between split broods with both homo- and heterozygous fathers and split broods where both fathers were heterozygous. Relative difference was higher in the former compared to the latter: Mann-Whitney: Z=−2.11, P=0.035 (nhomo/hetero=12, nhetero/hetero=19).
Allele-specific effects was tested for by comparing the fertilization success of the male with at least one copy of the allele in question with the fertilization success of the other male in the split brood. Split broods where both males had a copy of the allele in question were not included in the analysis. The results of these pairwise comparisons for each allele are summarized in Table 3. Only the allele t3 was significantly associated with increased fertilization success (Wilcoxon signed rank: Z=−2.05, P=0.04, see Fig. 3). We tested for effects of rareness of alleles on fertilization success in two ways. First, there was no correlation between the differences in RI-value between the two males in a split brood and the corresponding difference in fertilization success (Kendall rank correlation: tau=−0.58, P=0.65, n=36). Furthermore, within each split brood, the male with the highest RI-value did not have higher or lower fertilization success compared to the other male (Wilcoxon signed rank: Z=−0.47, P=0.62, n=36 pairs).
Average fertilization success in split broods with a heterozygous mother was not higher than in those with a homozygous mother (Mann-Whitney: Z=0.00, P=0.99, n=18). There was no significant relationship between how many MHC alleles a male shared with the female in the brood, i.e., the MHC similarity index, and fertilization success (Kendall rank correlation: tau=0.22, P=0.14, n=30).
Discussion
When given access to the same amount of eggs from the same female, sperm from MHC-heterozygous males fertilized more eggs than the same amount of sperm from homozygous males. Furthermore, there were larger differences in fertilization success in split broods that had MHC-homozygous and -heterozygous fathers, compared to split broods where both fathers were heterozygous. This suggests that male MHC-heterozygosity influences fertilization success. We see three likely explanations for these results.
First, the observed difference in fertilization success between MHC-homozygous and heterozygous males can be explained if heterozygous males simply have sperm that are better at reaching the egg than the sperm of homozygous males. Studies on inbreeding in laboratory and livestock animals have shown that genomic homozygosity adversely affects spermatogenesis (e.g., Rice et al. 1967; Wyrobek 1979), and this relationship has also been found in wild populations (e.g., Wildt et al. 1987). In the current study, however, we found no difference between MHC-heterozygous and homozygous males in percentage motile sperm or on sperm swimming speed, two measures of sperm quality thought to be important in determining fertilization success. It therefore seems unlikely that the observed differences in fertilization success between homo- and heterozygous males can be explained by differences in their sperm’s ability to reach the egg.
Second, eggs that fail to reach the eye-ova stage may, despite being fertilized, die during development. Therefore, rather than reflecting differences in fertilization success, the difference in number of eggs reaching the eye-ova stage may reflect differences in embryo survival between eggs fertilized by homo- and heterozygous males. Thus, we cannot rule out the possibility that the observed differences in eggs reaching the eye-ova stage between MHC-heterozygous and homozygous males actually indicate higher survival by eggs fertilized by heterozygous males under our experimental procedure. However, why eggs fertilized by MHC-homozygous males should have higher mortality is unclear. In species with parental care, abortional selection against MHC-homozygous embryos may serve an adaptive role as part of cryptic female choice (Eberhard 1996), but the evidence for this is mixed (Apanius et al. 1997). Regardless, abortional selection cannot be an adaptive mechanism of cryptic female choice in the Arctic charr, since, at spawning, the female has completed her reproductive investment. Thus, apart from the unlikely event of egg or sibling competition for limited resources, any embryo mortality would in this system be mal-adaptive.
The third explanation is that eggs choose sperm based on some phenotypically expressed indicator or correlate of MHC-heterozygosity. Paternal genes in the spermatogenic sections of the testes most likely produce the sperm phenotype, as the haploid genome of the sperm remains unexpressed until after its fusion with the egg (Cohen 1991). Thus, it seems likely that any egg choice of sperm is based on phenotypic markers such as, for example, olfactory cues (Ziegler et al. 2002), from the diploid paternal origin of the sperm. However, if sperm choice based on some diploid originated cues should be adaptive, these cues must relate to some qualities of the haploid genome of the sperm cells. The excess homozygosity and comparatively low allele count may indicate that this Arctic charr population has either gone through a recent bottleneck or experienced extensive inbreeding, which is not a surprise, given the complex history of post-glacial Arctic charr colonization of the lakes in northern Norway (Jonsson and Jonsson 2001). This suggests that the Arctic charr in this lake should be experiencing out-breeding selection through, for example, female choice for dissimilar alleles to ensure compatibility between paternal and maternal genotype. Urbach and co-workers (unpublished data) showed that the ovarian fluid of the Arctic charr might impose adverse effects on the motility of sperm from males that share alleles with the female compared to males with no shared alleles. This effect of shared alleles on sperm motility does perhaps not translate into differences in fertilization success, as fertilization success in our study is not related to female heterozygosity nor is it related to our MHC similarity index. However, variation between females in how their ovarian fluid stimulates sperm movement might explain the relatively large variation between average fertilization success between split broods (Urbach et al. 2004). Nevertheless, based on the findings in this study, fertilization success seems to be independent of the female genotype. Thus, the effect of heterozygosity on fertilization success seems not to be a mechanism to facilitate out-breeding.
As an alternative to out-breeding associated preferences, the comparatively limited MHC variation and high level of homozygosity in our sample may indicate directional selection for certain MHC-alleles. For example, if the eggs have a higher or lower affinity for sperm cells with certain alleles, then heterozygous males may have a higher chance of falling within this affinity. In this scenario, eggs of individual females are actually selecting for certain MHC-alleles and simply because MHC-heterozygous males have more “lottery tickets” in this “non-random lottery”, they will be at an advantage. According to models of parasite-host co-evolution, such preferred alleles should be relatively rare, but allele rareness is not related to fertilization success in this study. However, we still find limited support for allele-specific effects on fertilization success, as males with the t3-allele tend to fertilize more than the other male in the split brood. Furthermore, the t3-allele is also found more often in heterozygous compared to homozygous males (21% of heterozygous males compared to 10% of the homozygous males had the t3 allele). Thus, eggs may be tuned towards obtaining sperm with specific individual MHC-alleles. Given the immunological significance of the MHC, it is natural to interpret this finding within the context of good genes models of sexual selection. Consequently, this study documents the presence of cryptic female choice, potentially allowing females to obtain male gametes with alleles that can offer superior parasite resistance against currently pathogenic parasites. However, as our study was conducted in a natural population, we have no information on other aspects of the genetic variation in the population. Thus, the results observed may be influenced by other loci, and not exclusively by the investigated MHC-locus. In conclusion, our findings can be seen to support the idea that female choice can include sperm selection by the ovum, and that the criteria used in this process include spermatozoal MHC-genotype.
References
Amanze D, Iyengar A (1990) The micropyle: a sperm guidance system in teleost fertilization. Development 109:495–500
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Apanius V, Penn D, Slev PR, Ruff LR, Potts W (1997) The nature of selection on the major histocompatibility complex. Crit Rev Immunol 17:179–224
Birkhead TR, Møller AP (1998) Sperm competition and sexual selection. Academic, London
Birkhead TR, Pizzari T (2002) Postcopulatory sexual selection. Nat Rev Genet 3:262–273
Carré D, Sardet C (1984) Fertilization and early development in Beroe ovata. Dev Biol 105:188–195
Cohen J (1991) The case for and against sperm selection. In: Bacetti B (ed) Comparative spermatology 20 years after. Raven, New York, pp 759–764
Eberhard WG (1996) Female control: sexual selection by cryptic female choice. Princeton University Press, Princeton
Egid K, Brown JL (1989) The major histocompatibility complex and female mating preferences in mice. Anim Behav 38:548–550
Fisher S, Lerman LS (1983) DNA fragments differing by single base-pair substitutions are separated in denaturing gradient gels: correspondence with melting theory. Proc Natl Acad Sci 80:1579–1583
Hamilton WD, Poulin R (1997) The Hamilton and Zuk hypothesis revisited: a meta-analytical approach. Behaviour 134:299–320
Hamilton WD, Zuk M (1982) Heritable true fitness and bright birds: a role for parasites? Science 218:384–387
Janeway CA, Travers P (1997) Immunobiology: the immune system in health and disease. Garland, New York
Johnson L (1980) The Arctic charr, Salvelinus alpinus. In: Balon EK (ed) Charrs, salmonid fishes of the genus Salvelinus. Junk, The Hague, pp 15–98
Jonsson B, Jonsson N (2001) Polymorphism and speciation in the Arctic charr. J Fish Biol 58:605–638
Jordan WC, Bruford MW (1998) New perspectives on mate choice and the MHC. Heredity 81:239–245
Kamler E (1992) Early life history of fish: an energetics approach. Chapman & Hall, London
Laird PW, Zijderveld A, Linders K, Rudnicki MA, Jaenisch R, Berns A (1991) Simplified mammalian DNA isolation procedure. Nucl Acid Res 19:4293
Langefors Å, Lohm J, von Schantz T, Grahn M (2000) Screening of Mhc variation in Atlantic salmon (Salmo salar): a comparison of restriction fragment length polymorphism (RFLP), denaturing gradient gel electrophoresis (DGGE) and sequencing. Mol Ecol 9:215–219
Miller KM, Withler RE (1996) Sequence analysis of a polymorphic Mhc class II gene in Pacific salmon. Immunogenetics 43:337–351
Miller KM, Withler RE (1997) Mhc diversity in Pacific salmon: population structure and trans-species allelism. Hereditas 127:83–95
Myers RM, Maniatis T, Lerman LS (1987) Detection and localization of single base changes by denaturing gradient gel electrophoresis. Methods Enzymol 155:501–527
Ober C, Weitkamp LR, Cox N, Dytch H, Kostyu D, Elias S (1994) HLA and mate choice in humans. Am J Hum Genet 61:497–504
Olsén KH, Grahn M, Lohm J, Langefors Å (1998) MHC and kin discrimination in juvenile Arctic charr, Salvelinus alpinus (L.). Anim Behav 56:319–327
Penn DJ (2002) The scent of genetic compatibility: sexual selection and the major histocompatibility complex. Ethology 108:1–21
Potts WK, Manning CJ, Wakeland EK (1991) Mating patterns in seminatural populations of mice influenced by MHC genotype. Nature 352:619–621
Raymond M, Rousset F (1995) GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J Hered 86:248–249
Rice VA, Andrews FN, Warwick EJ, Legates JE (1967) Breeding and improvement of farm animals. McGraw-Hill, New York
Rülicke T, Chapuisat M, Homberger FR, Macas E, Wedekind C (1998) MHC-genotype of progeny influenced by parental infection. Proc R Soc Lond B 265:711–716
Schantz T von, Wittzell H, Göransson G, Grahn M, Persson K (1996) MHC genotype and male ornamentation: genetic evidence for the Hamilton-Zuk model. Proc R Soc Lond B 263:265–271
Siva-Jothy MT, Skarstein F (1998) Towards a functional understanding of “good genes”. Ecol Lett 1:178–185
Stockley P (1997) No evidence of sperm selection by female in common shrews. Proc R Soc Lond B 264:1497–1500
Tregenza T, Wedell N (2000) Genetic compatibility, mate choice and patterns of parentage: invited review. Mol Ecol 9:1013–1027
Urbach D, Folstad I, Rudolfsen G (2004) Sperm motility in ovarian fluid: Cryptic female choice in arctic charr? Behav Ecol Sociobiol (in press)
Wedekind C, Folstad I (1994) Adaptive and non-adaptive immunosuppression by sex hormones. Am Nat 143:936–938
Wedekind C, Furi S (1997) Body odour preferences in men and women: do they aim for specific MHC combinations or simply heterozygosity? Proc R Soc Lond B 264:1471–1479
Wedekind C, Seebeck T, Bettens F, Paepke AJ (1995) MHC-dependent mate preferences in humans. Proc R Soc Lond B 260:245–249
Wildt DE, Bush M, Goodrowe KL, Packer C, Pusey AE, Brown JL, Joslin P, O’Brien SJ (1987) Reproductive and genetic consequences of founding isolated populations. Nature 329:328–331
Wilson N, Tubman SC, Eady PE, Robertson GW (1997) Female genotype affects male success in sperm competition. Proc R Soc Lond B 264:1491–1495
Wyrobek AJ (1979) Changes in mammalian sperm morphology after x-ray and chemical exposures. Genetics 92:105–119
Yamazaki K, Boyse EA, Miké V, Mathieson BJ, Abbot BJ, Boyse J, Zayas ZA (1976) Control of mating preferences in mice by genes in the major histocompatibility complex. J Exp Med 144:1324–1335
Ziegler A, Dohr G, Uchanska-Ziegler B (2002) Possible roles for products of polymorphic MHC and linked olfactory receptor genes during selection processes in reproduction. Am J Reprod Immunol 48:34–42
Acknowledgements
We are grateful for the constructive comments of Claus Wedekind, Jakob Lohm and several anonymous referees, and the patience of editor Mark Abrahams.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Abrahams
Rights and permissions
About this article
Cite this article
Skarstein, F., Folstad, I., Liljedal, S. et al. MHC and fertilization success in the Arctic charr (Salvelinus alpinus). Behav Ecol Sociobiol 57, 374–380 (2005). https://doi.org/10.1007/s00265-004-0860-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-004-0860-z