Abstract
Purpose
Loose fragments in osteochondritis dissecans (OCD) of the knee require internal fixation. On the other hand, loose fragments derived from spontaneous osteonecrosis of the knee (SONK) are usually removed. However, the difference in healing potential between OCD- and SONK-related loose fragments has not been elucidated. In this study, we investigated proliferative activity and redifferentiation potential of normal cartilage-derived and loose fragment-derived chondrocytes.
Methods
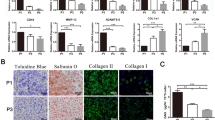
Cells were prepared from normal articular cartilages and loose fragment cartilages derived from knee OCD and SONK. Cellular proliferation was compared. Redifferentiation ability of pellet-cultured chondrocytes was assessed by real-time PCR analyses. Mesenchymal differentiation potential was investigated by histological analyses. Positive ratio of a stem cell marker CD166 was evaluated in each cartilaginous tissue.
Results
Normal and OCD chondrocytes showed a higher proliferative activity than SONK chondrocytes. Chondrogenic pellets derived from normal and OCD chondrocytes produced a larger amount of safranin O-stained proteoglycans compared with SONK-derived pellets. Expression of chondrogenic marker genes was inferior in SONK pellets. The CD166-positive ratio was higher in normal cartilages and OCD loose fragments than in SONK loose fragments.
Conclusions
The OCD chondrocytes maintained higher proliferative activity and redifferentiation potential compared with SONK chondrocytes. Our results suggest that chondrogenic properties of loose fragment-derived cells and the amount of CD166-positive cells may affect the repair process of osteochondral defects.




Similar content being viewed by others
References
Kocher MS, Tucker R, Ganley TJ, Flynn JM (2006) Management of osteochondritis dissecans of the knee: current concepts review. Am J Sports Med 34:1181–1191
Bradley J, Dandy DJ (1989) Osteochondritis dissecans and other lesions of the femoral condyles. J Bone Joint Surg Br 71:518–522
Mizuta H, Nakamura E, Otsuka Y, Kudo S, Takagi K (2001) Osteochondritis dissecans of the lateral femoral condyle following total resection of the discoid lateral meniscus. Arthroscopy 17:608–612
Uozumi H, Sugita T, Aizawa T, Takahashi A, Ohnuma M, Itoi E (2009) Histologic findings and possible causes of osteochondritis dissecans of the knee. Am J Sports Med 37:2003–2008
Adachi N, Motoyama M, Deie M, Ishikawa M, Arihiro K, Ochi M (2009) Histological evaluation of internally-fixed osteochondral lesions of the knee. J Bone Joint Surg Br 91:823–829
Patel DV, Breazeale NM, Behr CT, Warren RF, Wickiewicz TL, O’Brien SJ (1998) Osteonecrosis of the knee: current clinical concepts. Knee Surg Sports Traumatol Arthrosc 6:2–11
Yamamoto T, Bullough PG (2000) Spontaneous osteonecrosis of the knee: the result of subchondral insufficiency fracture. J Bone Joint Surg Am 82:858–866
Kraenzlin ME, Graf C, Meier C, Kraenzlin C, Friedrich NF (2010) Possible beneficial effect of bisphosphonates in osteonecrosis of the knee. Knee Surg Sports Traumatol Arthrosc 18:1638–1644
Koshino T (1982) The treatment of spontaneous osteonecrosis of the knee by high tibial osteotomy with and without bone-grafting or drilling of the lesion. J Bone Joint Surg Am 64:47–58
Takata N, Furumatsu T, Abe N, Naruse K, Ozaki T (2011) Comparison between loose fragment chondrocytes and condyle fibrochondrocytes in cellular proliferation and redifferentiation. J Orthop Sci 16:589–597
Pascual-Garrido C, Tanoira I, Muscolo DL, Ayerza MA, Makino A (2010) Viability of loose body fragments in osteochondritis dissecans of the knee. A series of cases. Int Orthop 34:827–831
Garvican ER, Vaughan-Thomas A, Redmond C, Clegg PD (2008) Chondrocytes harvested from osteochondritis dissecans cartilage are able to undergo limited in vitro chondrogenesis despite having perturbations of cell phenotype in vivo. J Orthop Res 26:1133–1140
Brittberg M, Winalski CS (2003) Evaluation of cartilage injuries and repair. J Bone Joint Surg Am 85(Suppl 2):58–69
Date H, Furumatsu T, Sakoma Y, Yoshida A, Hayashi Y, Abe N, Ozaki T (2010) GDF-5/7 and bFGF activate integrin α2-mediated cellular migration in rabbit ligament fibroblasts. J Orthop Res 28:225–231
Saiga K, Furumatsu T, Yoshida A, Masuda S, Takihira S, Abe N, Ozaki T (2010) Combined use of bFGF and GDF-5 enhances the healing of medial collateral ligament injury. Biochem Biophys Res Commun 402:329–334
Furumatsu T, Hachioji M, Saiga K, Takata N, Yokoyama Y, Ozaki T (2010) Anterior cruciate ligament-derived cells have high chondrogenic potential. Biochem Biophys Res Commun 391:1142–1147
Furumatsu T, Tsuda M, Taniguchi N, Tajima Y, Asahara H (2005) Smad3 induces chondrogenesis through the activation of SOX9 via CREB-binding protein/p300 recruitment. J Biol Chem 280:8343–8350
Grogan SP, Barbero A, Winkelmann V, Rieser F, Fitzsimmons JS, O’Driscoll S, Martin I, Mainil-Varlet P (2006) Visual histological grading system for the evaluation of in vitro-generated neocartilage. Tissue Eng 12:2141–2149
Matsumoto E, Furumatsu T, Kanazawa T, Tamura M, Ozaki T (2012) ROCK inhibitor prevents the dedifferentiation of human articular chondrocytes. Biochem Biophys Res Commun 420:124–129
Furumatsu T, Kanazawa T, Yokoyama Y, Abe N, Ozaki T (2011) Inner meniscus cells maintain higher chondrogenic phenotype compared with outer meniscus cells. Connect Tissue Res 52:459–465
Pretzel D, Linss S, Rochler S, Endres M, Kaps C, Alsalameh S, Kinne RW (2011) Relative percentage and zonal distribution of mesenchymal progenitor cells in human osteoarthritic and normal cartilage. Arthritis Res Ther 13:R64
Benya PD, Shaffer JD (1982) Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 30:215–224
Adkisson HD, Gillis MP, Davis EC, Maloney W, Hruska KA (2001) In vitro generation of scaffold independent neocartilage. Clin Orthop Relat Res 391:S280–S294
Barbero A, Grogan S, Schäfer D, Heberer M, Mainil-Varlet P, Martin I (2004) Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity. Osteoarthr Cartil 12:476–484
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Alsalameh S, Amin R, Gemba T, Lotz M (2004) Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum 50:1522–1532
Caplan AI, Dennis JE (2006) Mesenchymal stem cells as trophic mediators. J Cell Biochem 98:1076–1084
Chen CC, Liao CH, Wang YH, Hsu YM, Huang SH, Chang CH, Fang HW (2012) Cartilage fragments from osteoarthritic knee promote chondrogenesis of mesenchymal stem cells without exogenous growth factor induction. J Orthop Res 30:393–400
Furumatsu T, Shukunami C, Amemiya-Kudo M, Shimano H, Ozaki T (2010) Scleraxis and E47 cooperatively regulate the Sox9-dependent transcription. Int J Biochem Cell Biol 42:148–156
Furumatsu T, Asahara H (2010) Histone acetylation influences the activity of Sox9-related transcriptional complex. Acta Med Okayama 64:351–357
Acknowledgments
We are grateful to Prof. Nobuhiro Abe, Ms. Emi Matsumoto, Ms. Aki Yoshida, and Ms. Reina Tanaka for their technical support. This work was supported by Grants from the Japan Society for the Promotion of Science (No. 24791546), the JSPS Fujita Memorial Fund for Medical Research, and the Nakatomi Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sakata, K., Furumatsu, T., Miyazawa, S. et al. Comparison between normal and loose fragment chondrocytes in proliferation and redifferentiation potential. International Orthopaedics (SICOT) 37, 159–165 (2013). https://doi.org/10.1007/s00264-012-1728-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-012-1728-x