Abstract.
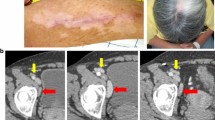
The objective of this study was to determine the safety and antitumor activity of an autologous GM-CSF-secreting melanoma cell vaccine that was engineered ex vivo with recombinant replication-incompetent adenovirus harboring a human GM-CSF gene (Adv/hGM-CSF). Melanoma samples were surgically obtained from 30 patients (15 female and 15 male, ages ranging from 23 to 87) and were processed for vaccine preparation. Due to stringent eligibility criteria, 9 out of 30 patients were enrolled in the phase 1 clinical trial (FDA IND7677). Melanoma cell lines established from surgical specimens of 9 patients were transduced with Adv/hGM-CSF (MOI of 100) and subsequently irradiated at 35 Gy. These cell lines secreted human GM-CSF in vitro at an average rate of 80–424 ng/106 cells/24 h. All patients were intradermally and subcutaneously injected at several sites with irradiated autologous melanoma cells (2×106–1×107 in 300 µl saline), 2–10 times, at intervals of 4–8 weeks. None of the patients vaccinated showed any serious adverse systemic response. Three patients (nos.1, 6 and 7) demonstrated local reaction (erythema) to the vaccination. Tumor-specific CTL assays performed in the absence of K562 cells showed that the levels of CTLs in peripheral blood of 5 patients increased following vaccination, whereas those in one patient declined. Levels of CTLs assayed in the presence of K562 cells were considerably lower than those assayed in the absence of K562 cells, but were also found to increase following vaccination in the peripheral blood of 6 patients. A patient who had been vaccinated 10 times (patient 1) responded to the vaccination by apparent reduction in size of metastatic tumor in the lung. Immunohistochemical examination of the vaccination sites of patient 1, biopsied after the 3rd and 4th vaccination, showed that the vaccination sites responded with infiltration of inflammatory cells, such as T cells (CD3+, CD8+), macrophages and dendritic cells (CD83+), for a period up to about 8 days. These data suggest that repeated vaccinations with irradiated autologous GM-CSF-producing tumor cells were well tolerated by patients and led to the activation of an antitumor immune response in some patients.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Kusumoto, M., Umeda, S., Ikubo, A. et al. Phase 1 clinical trial of irradiated autologous melanoma cells adenovirally transduced with human GM-CSF gene. Cancer Immunol Immunother 50, 373–381 (2001). https://doi.org/10.1007/s002620100213
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s002620100213