Abstract
Background
Patients with small-cell lung cancer (SCLC) have a dismal prognosis with limited overall survival (OS) despite a high response rate to chemotherapy. Recently, immune checkpoint inhibitors, combined with chemotherapy, as the first-line treatment for extensive-stage (ES)-SCLC have shown improvement in clinical outcomes.
Patients and methods
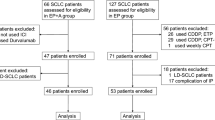
Real-world data from 68 Korean ES-SCLC patients, treated with atezolizumab, etoposide, and carboplatin at Yonsei Cancer Center between June 2019 and November 2020, were retrospectively analyzed to determine safety and efficacy using Cox regression analysis.
Results
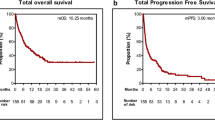
The median follow-up was 11.6 months. The median progression-free survival was 4.6 months (95% confidence interval [CI] 4.0–5.2), and the median OS was 12.0 months (95% CI 7.4–16.6). Baseline bone metastasis, immune-related adverse events (IRAEs), and elevated LDH were related to OS (hazard ratio 2.18, 0.33, and 4.64; P = 0.05, 0.02, and 0.003, respectively). Among the 42 patients with disease progression, liver metastasis progression and baseline bone metastasis were associated with inferior OS, but without statistical significance (hazard ratio 2.47 and 1.97; P = 0.25 and 0.26, respectively). Overall, 61 (89.7%) patients experienced treatment-related adverse events (TRAEs), with hematologic toxicities as the most common grade 3–4 TRAEs. Twenty-two (32.4%) patients experienced IRAEs, with skin rash as the most common, and five (7.4%) patients had grade-3 IRAEs (pneumonitis, hyperglycemia, and aspartate aminotransferase elevation).
Conclusion
Atezolizumab, combined with etoposide and carboplatin, showed efficacy and safety in our real-world data. Further studies are needed to predict the response to immunotherapy in SCLC.



Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Code availability
Not applicable.
Abbreviations
- CRT:
-
Concurrent chemoradiation therapy
- CI:
-
Confidence interval
- CR:
-
Complete response
- ES-SCLC:
-
Extensive-stage small-cell lung cancer
- HR:
-
Hazard ratio
- ICI:
-
Immune checkpoint inhibitor
- IRAE:
-
Immune-related adverse event
- mPFS:
-
Median progression-free survival
- mOS:
-
Median overall survival
- NSCLC:
-
Non-small cell lung cancer
- ORR:
-
Objective response rate
- OS:
-
Overall survival
- PD-1:
-
Programmed death protein-1
- PD-L1:
-
Programmed death-ligand 1
- PFS:
-
Progression-free survival
- PR:
-
Partial response
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- SCLC:
-
Small-cell lung cancer
- TRAE:
-
Treatment-related adverse event
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Barta JA, Powell CA, Wisnivesky JP (2019) Global epidemiology of lung cancer. Ann Glob Health. https://doi.org/10.5334/aogh.2419
Farago AF, Keane FK (2018) Current standards for clinical management of small cell lung cancer. Transl Lung Cancer Res 7(1):69–79. https://doi.org/10.21037/tlcr.2018.01.16
Schabath MB, Nguyen A, Wilson P, Sommerer KR, Thompson ZJ, Chiappori AA (2014) Temporal trends from 1986 to 2008 in overall survival of small cell lung cancer patients. Lung Cancer 86(1):14–21. https://doi.org/10.1016/j.lungcan.2014.07.014
Ready N, Farago AF, de Braud F, Atmaca A, Hellmann MD, Schneider JG et al (2019) Third-line nivolumab monotherapy in recurrent SCLC: checkmate 032. J Thorac Oncol 14(2):237–244. https://doi.org/10.1016/j.jtho.2018.10.003
Ott PA, Elez E, Hiret S, Kim DW, Morosky A, Saraf S, Piperdi B, Mehnert JM (2017) Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the phase Ib KEYNOTE-028 study. J Clin Oncol 35(34):3823–3829. https://doi.org/10.1200/jco.2017.72.5069
Chung HC, Piha-Paul SA, Lopez-Martin J, Schellens JHM, Kao S, Miller WH Jr et al (2020) Pembrolizumab after two or more lines of previous therapy in patients with recurrent or metastatic SCLC: results from the KEYNOTE-028 and KEYNOTE-158 studies. J Thorac Oncol 15(4):618–627. https://doi.org/10.1016/j.jtho.2019.12.109
Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ et al (2018) First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 379(23):2220–2229. https://doi.org/10.1056/NEJMoa1809064
Mansfield AS, Każarnowicz A, Karaseva N, Sánchez A, De Boer R, Andric Z et al (2020) Safety and patient-reported outcomes of atezolizumab, carboplatin, and etoposide in extensive-stage small-cell lung cancer (IMpower133): a randomized phase I/III trial. Ann Oncol 31(2):310–317. https://doi.org/10.1016/j.annonc.2019.10.021
Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D et al (2019) Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 394(10212):1929–1939. https://doi.org/10.1016/s0140-6736(19)32222-6
Goldman JW, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D et al (2020) Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. https://doi.org/10.1016/s1470-2045(20)30539-8
Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csőszi T et al (2020) Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol 38(21):2369–2379. https://doi.org/10.1200/jco.20.00793
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Common Terminology Criteria for Adverse Events (CTCAE), version 4.0 (2009) National Cancer Institute. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed 28 May 2009
Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F et al (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378(22):2078–2092. https://doi.org/10.1056/NEJMoa1801005
Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J et al (2018) Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379(21):2040–2051. https://doi.org/10.1056/NEJMoa1810865
Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G, Jr. et al (2019) Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 394(10212):1915–1928. https://doi.org/10.1016/s0140-6736(19)32591-7
Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H et al (2018) Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 379(22):2108–2121. https://doi.org/10.1056/NEJMoa1809615
Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D et al (2019) Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380(12):1116–1127. https://doi.org/10.1056/NEJMoa1816714
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY et al (2020) Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 382(20):1894–1905. https://doi.org/10.1056/NEJMoa1915745
Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B et al (2021) Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 384(9):829–841. https://doi.org/10.1056/NEJMoa2026982
Hamilton G, Rath B (2019) Immunotherapy for small cell lung cancer: mechanisms of resistance. Expert Opin Biol Ther 19(5):423–432. https://doi.org/10.1080/14712598.2019.1592155
Galluzzi L, Humeau J, Buqué A, Zitvogel L, Kroemer G (2020) Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol 17(12):725–741. https://doi.org/10.1038/s41571-020-0413-z
Golden EB, Frances D, Pellicciotta I, Demaria S, Helen Barcellos-Hoff M, Formenti SC (2014) Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology 3:e28518. https://doi.org/10.4161/onci.28518
Nakazawa K, Kurishima K, Tamura T, Kagohashi K, Ishikawa H, Satoh H, Hizawa N (2012) Specific organ metastases and survival in small cell lung cancer. Oncol Lett 4(4):617–620. https://doi.org/10.3892/ol.2012.792
Landi L, D’Incà F, Gelibter A, Chiari R, Grossi F, Delmonte A et al (2019) Bone metastases and immunotherapy in patients with advanced non-small-cell lung cancer. J Immunother Cancer 7(1):316. https://doi.org/10.1186/s40425-019-0793-8
Ricciuti B, Genova C, De Giglio A, Bassanelli M, Dal Bello MG, Metro G, Brambilla M, Baglivo S, Grossi F, Chiari R (2019) Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol 145(2):479–485. https://doi.org/10.1007/s00432-018-2805-3
Grangeon M, Tomasini P, Chaleat S, Jeanson A, Souquet-Bressand M, Khobta N et al (2019) Association between immune-related adverse events and efficacy of immune checkpoint inhibitors in non-small-cell lung cancer. Clin Lung Cancer 20(3):201–207. https://doi.org/10.1016/j.cllc.2018.10.002
Sato K, Akamatsu H, Murakami E, Sasaki S, Kanai K, Hayata A et al (2018) Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer 115:71–74. https://doi.org/10.1016/j.lungcan.2017.11.019
Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R et al (2018) Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol 4(3):374–378. https://doi.org/10.1001/jamaoncol.2017.2925
Zhang Z, Li Y, Yan X, Song Q, Wang G, Hu Y, Jiao S, Wang J (2019) Pretreatment lactate dehydrogenase may predict outcome of advanced non small-cell lung cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Cancer Med 8(4):1467–1473. https://doi.org/10.1002/cam4.2024
Zhang X, Guo M, Fan J, Lv Z, Huang Q, Han J, Wu F, Hu G, Xu J, Jin Y (2016) Prognostic significance of serum LDH in small cell lung cancer: A systematic review with meta-analysis. Cancer Biomark 16(3):415–423. https://doi.org/10.3233/cbm-160580
Schultheis AM, Scheel AH, Ozretić L, George J, Thomas RK, Hagemann T, Zander T, Wolf J, Buettner R (2015) PD-L1 expression in small cell neuroendocrine carcinomas. Eur J Cancer 51(3):421–426. https://doi.org/10.1016/j.ejca.2014.12.006
Antonia SJ, López-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP et al (2016) Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 17(7):883–895. https://doi.org/10.1016/s1470-2045(16)30098-5
Rudin CM, Poirier JT, Byers LA, Dive C, Dowlati A, George J et al (2019) Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat Rev Cancer 19(5):289–297. https://doi.org/10.1038/s41568-019-0133-9
Gay CM, Stewart CA, Park EM, Diao L, Groves SM, Heeke S et al (2021) Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell 39(3):346-360.e347. https://doi.org/10.1016/j.ccell.2020.12.014
Yu J, Green MD, Li S, Sun Y, Journey SN, Choi JE et al (2021) Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med 27(1):152–164. https://doi.org/10.1038/s41591-020-1131-x
Acknowledgements
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
SL and MHH conceptualized and designed the study. SL, HSS, BCA, SML, HRK, BCC, and MHH were involved in data acquisition. SL and MHH analyzed and interpreted data. SL performed statistical analysis. The first draft of the manuscript was written by SL and MHH, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethics approval
The study was approved by the institutional review board (IRB) at Severance Hospital (IRB number 4–2020-1151), and all experiments were performed in accordance with the guidelines and regulations of IRB of Severance Hospital. This study was given a formal waiver for the need of consent by the institutional review board of Severance Hospital in view of the retrospective nature of the study.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The study provides real-world data of Korean small-cell lung cancer patients showing comparable outcomes with IMpower133 study, in which Asians were underrepresented. Bone metastasis and liver metastasis progression suggested a poor prognosis.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, S., Shim, H.S., Ahn, BC. et al. Efficacy and safety of atezolizumab, in combination with etoposide and carboplatin regimen, in the first-line treatment of extensive-stage small-cell lung cancer: a single-center experience. Cancer Immunol Immunother 71, 1093–1101 (2022). https://doi.org/10.1007/s00262-021-03052-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-021-03052-w