Abstract
Introduction
The human papillomavirus (HPV) encoded oncoproteins E6 and E7 are constitutively expressed in HPV-associated cancers, making them logical therapeutic targets. Intramuscular immunization of patients with HPV16 L2E7E6 fusion protein vaccine (TA-CIN) is well tolerated and induces HPV-specific cellular immune responses. Efficacy of PD-1 immune checkpoint blockade correlates with the level of tumor-infiltrating CD8 + T cells, yet most patients lack significant tumor infiltration of immune cells making immune checkpoint blockade suboptimal. We hypothesized that intratumoral vaccination with TA-CIN could increase the number of tumor-infiltrating CD8 + T cells, synergize with PD-1 blockade and result in better control of tumors compared with either PD-1 blockade or vaccination alone.
Methods
We examined the immunogenicity and antitumor effects of intratumoral vaccination with TA-CIN alone or in combination with PD-1 blockade in the TC-1 syngeneic murine tumor model expressing HPV16 E6/E7.
Results
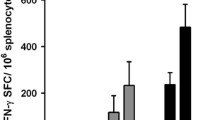
Intratumoral vaccination with TA-CIN induced stronger antigen-specific CD8 + T cell responses and antitumor effects. Intratumoral TA-CIN vaccination generated a systemic immune response that was able to control distal TC-1 tumors. Furthermore, intratumoral TA-CIN vaccination induced tumor infiltration of antigen-specific CD8 + T cells. Knockout of Batf3 abolished antigen-specific CD8 + T cell responses and antitumor effects of intratumoral TA-CIN vaccination. Finally, PD-1 blockade synergizes with intratumoral TA-CIN vaccination resulting in significantly enhanced antigen-specific CD8 + T cell responses and complete regression of tumors, whereas either alone failed to control established TC-1 tumor.
Conclusions
Our results provide rationale for future clinical testing of intratumoral TA-CIN vaccination in combination with PD-1 blockade for the control of HPV16-associated tumors.







Similar content being viewed by others
Data availability
Data generated from this study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
Parkin DM (2006) The global health burden of infection-associated cancers in the year 2002. Int J Cancer 118(12):3030–3044. https://doi.org/10.1002/ijc.21731
Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N (1999) Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 189(1):12–19. https://doi.org/10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F
Safaeian M, Solomon D, Castle PE (2007) Cervical cancer prevention–cervical screening: science in evolution. Obstet Gynecol Clin North Am 34(4):739–760. https://doi.org/10.1016/j.ogc.2007.09.004 ((ix))
Schiller JT, Lowy DR (2012) Understanding and learning from the success of prophylactic human papillomavirus vaccines. Nat Rev Microbiol 10(10):681–692. https://doi.org/10.1038/nrmicro2872
Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, Bratti MC, Schiller JT, Gonzalez P, Dubin G, Porras C, Jimenez SE, Lowy DR, Costa Rican HPVVTG (2007) Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA 298(7):743–753. https://doi.org/10.1001/jama.298.7.743
Markman M (2013) Chemoradiation in the management of cervix cancer: current status and future directions. Oncology 84(4):246–250. https://doi.org/10.1159/000346804
Roden RBS, Stern PL (2018) Opportunities and challenges for human papillomavirus vaccination in cancer. Nat Rev Cancer 18(4):240–254. https://doi.org/10.1038/nrc.2018.13
van der Burg SH, Kwappenberg KM, O’Neill T, Brandt RM, Melief CJ, Hickling JK, Offringa R (2001) Pre-clinical safety and efficacy of TA-CIN, a recombinant HPV16 L2E6E7 fusion protein vaccine, in homologous and heterologous prime-boost regimens. Vaccine 19(27):3652–3660. https://doi.org/10.1016/s0264-410x(01)00086-x
Yugawa T, Kiyono T (2009) Molecular mechanisms of cervical carcinogenesis by high-risk human papillomaviruses: novel functions of E6 and E7 oncoproteins. Rev Med Virol 19(2):97–113. https://doi.org/10.1002/rmv.605
Hitzeroth II, Passmore JA, Shephard E, Stewart D, Muller M, Williamson AL, Rybicki EP, Kast WM (2009) Immunogenicity of an HPV-16 L2 DNA vaccine. Vaccine 27(46):6432–6434. https://doi.org/10.1016/j.vaccine.2009.06.015
Wang JW, Hung CF, Huh WK, Trimble CL, Roden RB (2015) Immunoprevention of human papillomavirus-associated malignancies. Cancer Prev Res (Phila) 8(2):95–104. https://doi.org/10.1158/1940-6207.CAPR-14-0311
de Jong A, O’Neill T, Khan AY, Kwappenberg KM, Chisholm SE, Whittle NR, Dobson JA, Jack LC, St Clair Roberts JA, Offringa R, van der Burg SH, Hickling JK (2002) Enhancement of human papillomavirus (HPV) type 16 E6 and E7-specific T-cell immunity in healthy volunteers through vaccination with TA-CIN, an HPV16 L2E7E6 fusion protein vaccine. Vaccine 20(29–30):3456–3464. https://doi.org/10.1016/s0264-410x(02)00350-x
Daayana S, Elkord E, Winters U, Pawlita M, Roden R, Stern PL, Kitchener HC (2010) Phase II trial of imiquimod and HPV therapeutic vaccination in patients with vulval intraepithelial neoplasia. Br J Cancer 102(7):1129–1136. https://doi.org/10.1038/sj.bjc.6605611
Davidson EJ, Faulkner RL, Sehr P, Pawlita M, Smyth LJ, Burt DJ, Tomlinson AE, Hickling J, Kitchener HC, Stern PL (2004) Effect of TA-CIN (HPV 16 L2E6E7) booster immunisation in vulval intraepithelial neoplasia patients previously vaccinated with TA-HPV (vaccinia virus encoding HPV 16/18 E6E7). Vaccine 22(21–22):2722–2729. https://doi.org/10.1016/j.vaccine.2004.01.049
Massarelli E, William W, Johnson F, Kies M, Ferrarotto R, Guo M, Feng L, Lee JJ, Tran H, Kim YU, Haymaker C, Bernatchez C, Curran M, Zecchini Barrese T, Rodriguez Canales J, Wistuba I, Li L, Wang J, van der Burg SH, Melief CJ, Glisson B (2019) Combining immune checkpoint blockade and tumor-specific vaccine for patients with incurable human papillomavirus 16-related cancer: a phase 2 clinical trial. JAMA Oncol 5(1):67–73. https://doi.org/10.1001/jamaoncol.2018.4051
Mkrtichyan M, Chong N, Abu Eid R, Wallecha A, Singh R, Rothman J, Khleif SN (2013) Anti-PD-1 antibody significantly increases therapeutic efficacy of Listeria monocytogenes (Lm)-LLO immunotherapy. J Immunother Cancer 1:15. https://doi.org/10.1186/2051-1426-1-15
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12(4):252–264. https://doi.org/10.1038/nrc3239
Tang H, Wang Y, Chlewicki LK, Zhang Y, Guo J, Liang W, Wang J, Wang X, Fu YX (2016) Facilitating T cell infiltration in tumor microenvironment overcomes resistance to PD-L1 blockade. Cancer Cell 29(3):285–296. https://doi.org/10.1016/j.ccell.2016.02.004
Steele KE, Tan TH, Korn R, Dacosta K, Brown C, Kuziora M, Zimmermann J, Laffin B, Widmaier M, Rognoni L, Cardenes R, Schneider K, Boutrin A, Martin P, Zha J, Wiestler T (2018) Measuring multiple parameters of CD8+ tumor-infiltrating lymphocytes in human cancers by image analysis. J Immunother Cancer 6(1):20. https://doi.org/10.1186/s40425-018-0326-x
FDA approves pembrolizumab for advanced cervical cancer with disease progression during or after chemotherapy. (2018) fda.gov. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-advanced-cervical-cancer-disease-progression-during-or-after-chemotherapy. 2020
Hammerich L, Bhardwaj N, Kohrt HE, Brody JD (2016) In situ vaccination for the treatment of cancer. Immunotherapy 8(3):315–330. https://doi.org/10.2217/imt.15.120
Li H, Yu J, Wu Y, Shao B, Wei X (2020) In situ antitumor vaccination: targeting the tumor microenvironment. J Cell Physiol. https://doi.org/10.1002/jcp.29551
Nobuoka D, Yoshikawa T, Takahashi M, Iwama T, Horie K, Shimomura M, Suzuki S, Sakemura N, Nakatsugawa M, Sadamori H, Yagi T, Fujiwara T, Nakatsura T (2013) Intratumoral peptide injection enhances tumor cell antigenicity recognized by cytotoxic T lymphocytes: a potential option for improvement in antigen-specific cancer immunotherapy. Cancer Immunol Immunother 62(4):639–652. https://doi.org/10.1007/s00262-012-1366-6
Lin KY, Guarnieri FG, Staveley-O’Carroll KF, Levitsky HI, August JT, Pardoll DM, Wu TC (1996) Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Can Res 56(1):21–26
Marabelle A, Kohrt H, Caux C, Levy R (2014) Intratumoral immunization: a new paradigm for cancer therapy. Clin Cancer Res 20(7):1747–1756. https://doi.org/10.1158/1078-0432.CCR-13-2116
Sanchez-Paulete AR, Teijeira A, Cueto FJ, Garasa S, Perez-Gracia JL, Sanchez-Arraez A, Sancho D, Melero I (2017) Antigen cross-presentation and T-cell cross-priming in cancer immunology and immunotherapy. Ann Oncol 28(suppl_12):xii44–xii55. https://doi.org/10.1093/annonc/mdx237
Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, Iyer AK (2017) PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol 8:561. https://doi.org/10.3389/fphar.2017.00561
Fu J, Kanne DB, Leong M, Glickman LH, McWhirter SM, Lemmens E, Mechette K, Leong JJ, Lauer P, Liu W, Sivick KE, Zeng Q, Soares KC, Zheng L, Portnoy DA, Woodward JJ, Pardoll DM, Dubensky TW Jr, Kim Y (2015) STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci Transl Med 7(283):283ra252. https://doi.org/10.1126/scitranslmed.aaa4306
Fu J, Malm IJ, Kadayakkara DK, Levitsky H, Pardoll D, Kim YJ (2014) Preclinical evidence that PD1 blockade cooperates with cancer vaccine TEGVAX to elicit regression of established tumors. Can Res 74(15):4042–4052. https://doi.org/10.1158/0008-5472.Can-13-2685
Abdel-Motal UM, Wigglesworth K, Galili U (2009) Intratumoral injection of alpha-gal glycolipids induces a protective anti-tumor T cell response which overcomes Treg activity. Cancer Immunol Immunother 58(10):1545–1556. https://doi.org/10.1007/s00262-009-0662-2
Ishida E, Lee J, Campbell JS, Chakravarty PD, Katori Y, Ogawa T, Johnson L, Mukhopadhyay A, Faquin WC, Lin DT, Wirth LJ, Pierce RH, Pai SI (2019) Intratumoral delivery of an HPV vaccine elicits a broad anti-tumor immune response that translates into a potent anti-tumor effect in a preclinical murine HPV model. Cancer Immunol Immunother 68(8):1273–1286. https://doi.org/10.1007/s00262-019-02357-1
Newman JH, Chesson CB, Herzog NL, Bommareddy PK, Aspromonte SM, Pepe R, Estupinian R, Aboelatta MM, Buddhadev S, Tarabichi S, Lee M, Li S, Medina DJ, Giurini EF, Gupta KH, Guevara-Aleman G, Rossi M, Nowicki C, Abed A, Goldufsky JW, Broucek JR, Redondo RE, Rotter D, Jhawar SR, Wang SJ, Kohlhapp FJ, Kaufman HL, Thomas PG, Gupta V, Kuzel TM, Reiser J, Paras J, Kane MP, Singer EA, Malhotra J, Denzin LK, Sant’Angelo DB, Rabson AB, Lee LY, Lasfar A, Langenfeld J, Schenkel JM, Fidler MJ, Ruiz ES, Marzo AL, Rudra JS, Silk AW, Zloza A (2020) Intratumoral injection of the seasonal flu shot converts immunologically cold tumors to hot and serves as an immunotherapy for cancer. Proc Natl Acad Sci USA 117(2):1119–1128. https://doi.org/10.1073/pnas.1904022116
Sanchez-Paulete AR, Cueto FJ, Martinez-Lopez M, Labiano S, Morales-Kastresana A, Rodriguez-Ruiz ME, Jure-Kunkel M, Azpilikueta A, Aznar MA, Quetglas JI, Sancho D, Melero I (2016) Cancer immunotherapy with immunomodulatory anti-CD137 and anti-PD-1 monoclonal antibodies requires BATF3-dependent dendritic cells. Cancer Discov 6(1):71–79. https://doi.org/10.1158/2159-8290.CD-15-0510
Spranger S, Dai D, Horton B, Gajewski TF (2017) Tumor-residing BATF3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy. Cancer Cell 31(5):711–723. https://doi.org/10.1016/j.ccell.2017.04.003 ((e714))
Chandra J, Dutton JL, Li B, Woo WP, Xu Y, Tolley LK, Yong M, Wells JW, Leggatt GR, Finlayson N, Frazer IH (2017) DNA vaccine encoding HPV16 oncogenes E6 and E7 induces potent cell-mediated and humoral immunity which protects in tumor challenge and drives E7-expressing skin graft rejection. J Immunother (Hagerstown, Md: 1997) 40(2):62–70. https://doi.org/10.1097/cji.0000000000000156
Funding
This work was supported by the National Institutes of Health under award numbers R01CA237067 and P50CA098252.
Author information
Authors and Affiliations
Contributions
SP contributed to the design and conduction of the experiment. MT contributed to the interpretation of the data. Y-DL contributed to the performance of the experiments. MAC and EF contributed to the original draft preparation. LF, SG, and MT contributed to substantial review and preparation of the manuscript. RBSR contributed to the design of the study. C-FH contributed to the design of the study and interpretation of data. T-CW supervised the study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All procedures were performed under a prior-approved protocol of the Johns Hopkins Animal Care and Use Committee, and in accordance with recommendations for the proper use and care of laboratory animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Peng, S., Tan, M., Li, YD. et al. PD-1 blockade synergizes with intratumoral vaccination of a therapeutic HPV protein vaccine and elicits regression of tumor in a preclinical model. Cancer Immunol Immunother 70, 1049–1062 (2021). https://doi.org/10.1007/s00262-020-02754-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02754-x