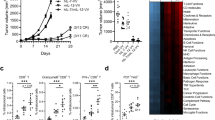
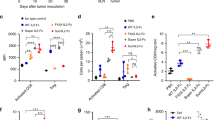
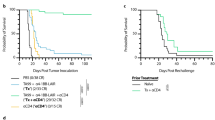
Abstract
High-dose IL-2 induces cancer regression but its therapeutic use is limited due to high toxicities resulting from its broad cell targeting. In one strategy to overcome this limitation, IL-2 has been modified to selectively target the intermediate affinity IL-2R that broadly activates memory-phenotypic CD8+ T and NK cells, while minimizing Treg-associated tolerance. In this study, we modeled an alternative strategy to amplify tumor antigen-specific TCR transgenic CD8+ T cells through limited application of a long-acting IL-2 fusion protein, mIL-2/mCD25, which selectively targets the high-affinity IL-2R. Here, mice were vaccinated with a tumor antigen and high-dose mIL-2/mCD25 was applied to coincide with the induction of the high affinity IL-2R on tumor-specific T cells. A single high dose of mIL-2/mCD25, but not an equivalent amount of IL-2, amplified the frequency and function of tumor-reactive CD8+ T effector (Teff) and memory cells. These mIL-2/mCD25-dependent effects relied on distinctive requirements for TLR signals during priming of CD8+ tumor-specific T cells. The mIL-2/mCD25-amplified tumor-reactive effector and memory T cells supported long-lasting antitumor responses to B16-F10 melanoma. This regimen only transiently increased Tregs, yielding a favorable Teff–Treg ratio within the tumor microenvironment. Notably, mIL-2/mCD25 did not increase non-tumor-specific Teff or NK cells within tumors, further substantiating the specificity of mIL-2/mCD25 for tumor antigen-activated T cells. Thus, the selectivity and persistence of mIL-2/mCD25 in conjunction with a tumor vaccine supports antitumor immunity through a mechanism that is distinct from recombinant IL-2 or IL-2-based biologics that target the intermediate affinity IL-2R.







Similar content being viewed by others
Availability of data and materials
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.
Code availability
Not applicable.
References
Rosenberg SA (2014) IL-2: the first effective immunotherapy for human cancer. J Immunol 192(12):5451–5458. https://doi.org/10.4049/jimmunol.1490019
Kohler PC, Sondel PM (1989) The role of interleukin-2 in cancer therapy. Cancer Surv 8(4):861–873
Boyman O, Sprent J (2012) The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol 12(3):180–190. https://doi.org/10.1038/nri3156
Malek TR, Castro I (2010) Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity 33(2):153–165. https://doi.org/10.1016/j.immuni.2010.08.004
Jiang T, Zhou C, Ren S (2016) Role of IL-2 in cancer immunotherapy. Oncoimmunology 5(6):e1163462. https://doi.org/10.1080/2162402X.2016.1163462
Kalia V, Sarkar S (2018) Regulation of effector and memory CD8 T cell differentiation by IL-2-A balancing Act. Front Immunol 9:2987. https://doi.org/10.3389/fimmu.2018.02987
Lotze MT, Frana LW, Sharrow SO, Robb RJ, Rosenberg SA (1985) In vivo administration of purified human interleukin 2. I. Half-life and immunologic effects of the Jurkat cell line-derived interleukin 2. J Immunol 134(1):157–166
Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, Paradise C, Kunkel L, Rosenberg SA (1999) High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 17(7):2105–2116. https://doi.org/10.1200/JCO.1999.17.7.2105
Kammula US, White DE, Rosenberg SA (1998) Trends in the safety of high dose bolus interleukin-2 administration in patients with metastatic cancer. Cancer 83(4):797–805
Shang B, Liu Y, Jiang SJ, Liu Y (2015) Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep 5:15179. https://doi.org/10.1038/srep15179
Sim GC, Martin-Orozco N, Jin L, Yang Y, Wu S, Washington E, Sanders D, Lacey C, Wang Y, Vence L, Hwu P, Radvanyi L (2014) IL-2 therapy promotes suppressive ICOS+ Treg expansion in melanoma patients. J Clin Invest 124(1):99–110. https://doi.org/10.1172/JCI46266
Boyman O, Arenas-Ramirez N (2019) Development of a novel class of interleukin-2 immunotherapies for metastatic cancer. Swiss Med Wkly 149:w14697. https://doi.org/10.4414/smw.2019.14697
Charych DH, Hoch U, Langowski JL, Lee SR, Addepalli MK, Kirk PB, Sheng D, Liu X, Sims PW, VanderVeen LA, Ali CF, Chang TK, Konakova M, Pena RL, Kanhere RS, Kirksey YM, Ji C, Wang Y, Huang J, Sweeney TD, Kantak SS, Doberstein SK (2016) NKTR-214, an engineered cytokine with biased IL2 receptor binding, increased tumor exposure, and marked efficacy in mouse tumor models. Clin Cancer Res 22(3):680–690. https://doi.org/10.1158/1078-0432.CCR-15-1631
Carmenate T, Pacios A, Enamorado M, Moreno E, Garcia-Martinez K, Fuente D, Leon K (2013) Human IL-2 mutein with higher antitumor efficacy than wild type IL-2. J Immunol 190(12):6230–6238. https://doi.org/10.4049/jimmunol.1201895
Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J (2006) Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science 311(5769):1924–1927. https://doi.org/10.1126/science.1122927
Ward NC, Yu A, Moro A, Ban Y, Chen X, Hsiung S, Keegan J, Arbanas JM, Loubeau M, Thankappan A, Yamniuk AP, Davis JH, Struthers M, Malek TR (2018) IL-2/CD25: a long-acting fusion protein that promotes immune tolerance by selectively targeting the IL-2 receptor on regulatory T cells. J Immunol 201(9):2579–2592. https://doi.org/10.4049/jimmunol.1800907
Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR (1994) T cell receptor antagonist peptides induce positive selection. Cell 76(1):17–27. https://doi.org/10.1016/0092-8674(94)90169-4
Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, Dellemijn TA, Antony PA, Spiess PJ, Palmer DC, Heimann DM, Klebanoff CA, Yu Z, Hwang LN, Feigenbaum L, Kruisbeek AM, Rosenberg SA, Restifo NP (2003) Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med 198(4):569–580. https://doi.org/10.1084/jem.20030590
Martins GA, Cimmino L, Liao J, Magnusdottir E, Calame K (2008) Blimp-1 directly represses Il2 and the Il2 activator Fos, attenuating T cell proliferation and survival. J Exp Med 205(9):1959–1965. https://doi.org/10.1084/jem.20080526
Oh S, Hwang ES (2014) The role of protein modifications of T-bet in cytokine production and differentiation of T helper cells. J Immunol Res 2014:589672. https://doi.org/10.1155/2014/589672
Hwang ES, Hong JH, Glimcher LH (2005) IL-2 production in developing Th1 cells is regulated by heterodimerization of RelA and T-bet and requires T-bet serine residue 508. J Exp Med 202(9):1289–1300. https://doi.org/10.1084/jem.20051044
Cui W, Joshi NS, Liu Y, Meng H, Kleinstein SH, Kaech SM (2014) TLR4 ligands lipopolysaccharide and monophosphoryl lipid a differentially regulate effector and memory CD8+ T Cell differentiation. J Immunol 192(9):4221–4232. https://doi.org/10.4049/jimmunol.1302569
Salem ML, Kadima AN, Cole DJ, Gillanders WE (2005) Defining the antigen-specific T-cell response to vaccination and poly(I:C)/TLR3 signaling: evidence of enhanced primary and memory CD8 T-cell responses and antitumor immunity. J Immunother 28(3):220–228. https://doi.org/10.1097/01.cji.0000156828.75196.0d
Rudra JS, Banasik BN, Milligan GN (2018) A combined carrier-adjuvant system of peptide nanofibers and toll-like receptor agonists potentiates robust CD8+ T cell responses. Vaccine 36(4):438–441. https://doi.org/10.1016/j.vaccine.2017.12.017
Kim HP, Kelly J, Leonard WJ (2001) The basis for IL-2-induced IL-2 receptor alpha chain gene regulation: importance of two widely separated IL-2 response elements. Immunity 15(1):159–172. https://doi.org/10.1016/s1074-7613(01)00167-4
Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R (2010) Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity 32(1):91–103. https://doi.org/10.1016/j.immuni.2009.11.010
McGlinchey RP, Shewmaker F, McPhie P, Monterroso B, Thurber K, Wickner RB (2009) The repeat domain of the melanosome fibril protein Pmel17 forms the amyloid core promoting melanin synthesis. Proc Natl Acad Sci U S A 106(33):13731–13736. https://doi.org/10.1073/pnas.0906509106
Castro I, Dee MJ, Malek TR (2012) Transient enhanced IL-2R signaling early during priming rapidly amplifies development of functional CD8+ T effector-memory cells. J Immunol 189(9):4321–4330. https://doi.org/10.4049/jimmunol.1202067
Overacre-Delgoffe AE, Vignali DAA (2018) Treg fragility: a prerequisite for effective antitumor immunity? Cancer Immunol Res 6(8):882–887. https://doi.org/10.1158/2326-6066.CIR-18-0066
Jacob JB, Kong YC, Nalbantoglu I, Snower DP, Wei WZ (2009) Tumor regression following DNA vaccination and regulatory T cell depletion in neu transgenic mice leads to an increased risk for autoimmunity. J Immunol 182(9):5873–5881. https://doi.org/10.4049/jimmunol.0804074
Tanaka A, Sakaguchi S (2017) Regulatory T cells in cancer immunotherapy. Cell Res 27(1):109–118. https://doi.org/10.1038/cr.2016.151
Smith M, Garcia-Martinez E, Pitter MR, Fucikova J, Spisek R, Zitvogel L, Kroemer G, Galluzzi L (2018) Trial Watch: toll-like receptor agonists in cancer immunotherapy. Oncoimmunology 7(12):e1526250. https://doi.org/10.1080/2162402X.2018.1526250
Acknowledgements
We thank Michael Dee and Aixin Yu for technical assistance, Mary Struthers and Francisco Ramirez-Valle at Bristol Myers Squibb for critically reading the manuscript, and Patricia Guevara, Jay Enten, and Shannon Saigh from the Flow Cytometry Core of the Sylvester Comprehensive Cancer Center (supported by NIH P30CA240139).
Funding
This research was supported by funding to T.R.M. from the NIH (R21CA195334) and Sylvester Comprehensive Cancer Center at the University of Miami.
Author information
Authors and Affiliations
Contributions
Conception and design: RH and TRM. Production and validation of mIL-2/mCD25: ASS. Acquisition of data: RH, KHT, JP, SH. Analysis and interpretation of data: RH and TRM. Manuscript Writing: RH and TRM. Revisions: All authors edited and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The University of Miami, T.R.M. and R.H. have patents pending on IL-2/CD25 fusion proteins (Wo2016022671A1;T.R.M) and their use (PCT/US20/13152; T.R.M., R.H) that have been licensed exclusively to Bristol Myers Squibb, and some research on IL-2/CD25 fusion proteins has been supported in part by a collaboration and sponsored research and licensing agreement with Bristol Myers Squibb. The other authors have no financial conflicts of interest.
Ethics approval
Animal studies were approved by the Institutional Animal Care and Use Committee at the University of Miami (Protocol 18-147).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hernandez, R., Toomer, K.H., Põder, J. et al. Sustained IL-2R signaling of limited duration by high-dose mIL-2/mCD25 fusion protein amplifies tumor-reactive CD8+ T cells to enhance antitumor immunity. Cancer Immunol Immunother 70, 909–921 (2021). https://doi.org/10.1007/s00262-020-02722-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02722-5