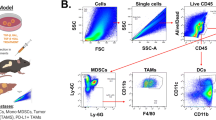
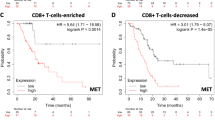
Abstract
The interactions between tumor immune microenvironment (TIME) and pancreatic cancer cells can affect chemotherapeutic efficacy; however, the mechanisms still remain largely unknown. Thirty items in TIME were comprehensively screened by using tissue microarray from pancreatic cancer patients. Their expressions, interconnections and predictive roles for survival were analyzed. Twenty-one of 30 items could stratify the survival of the patients; however, multivariate analysis found that only 5 independent risk factors could predict worse survival (M2-polarized tumor-associated macrophages (TAMs), IgG4 positive cells, TGF-β1, GM-CSF and lymphangiogenesis). They had a much higher expression levels in tumoral tissue, compared to peritumoral tissue. The Spearman analysis showed that M2-polarized TAM, TGF-β1 and GM-CSF were positively correlated with pancreatic cancer stem cells (PCSC), angiogenesis and lymphangiogenesis. Both human and murine pancreatic cancer cells could induce M2-polarized TAM, which showed substantial roles to decease chemotherapeutic effects. After treated by gemcitabine, both human and murine pancreatic cancer cell lines expressed higher level of immune check points, PCSC markers and varieties of immunosuppressive factors; however, TGF-β1 and GM-CSF had the highest increase. Based on the above results, TGF-β1 and GM-CSF were proposed to be the optimal potential targets to improve chemotherapeutic effects. In immunocompetent murine models, we demonstrated that combined blockade of TGF-β1 and GM-CSF improved the chemotherapeutic effects by inhibition of M2-polarized TAM and induction of CD8 positive T cells. This study presents a novel promising combined strategy to improve the chemotherapeutic effects for pancreatic cancer.






Similar content being viewed by others
Abbreviations
- ACT:
-
Adjuvant chemotherapy
- ARG1:
-
Arginase1
- CSF:
-
Colony stimulating factor
- CTL:
-
Cytotoxic T lymphocytes
- Gem:
-
Gemcitabine
- GM/M-CSF:
-
Granulocyte macrophage/macrophage colony stimulating factor
- IFN-γ:
-
Interferon-γ
- IL:
-
Interleukin
- iNOS:
-
Inducible nitric oxide synthase
- LPS:
-
Lipopolysaccharides
- MDSC:
-
Myeloid-derived suppressor cells
- MLVD:
-
Microlymphatic vascular density
- MVD:
-
Microvascular density
- PCSC:
-
Pancreatic cancer stem cells
- PDAC:
-
Pancreatic ductal adenocarcinoma
- PD-1:
-
Programmed death 1
- PDL-1:
-
Programmed death ligand-1
- STAT:
-
Signal transducer and activator of transcription
- TAM:
-
Tumor-associated macrophages
- TGF-β1:
-
Transforming growth factor beta-1
- Th1/2:
-
T helper 1/2
- TIME:
-
Tumor immune microenvironment
- TNF-α:
-
Tumor necrosis factor-α
- Treg:
-
Regulatory T cells
- VEGF:
-
Vascular endothelial growth factor
References
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68:7–30
Wu WM, Jin G, Wang CY et al (2019) The current surgical treatment of pancreatic cancer in China a national wide cross-sectional study. J Pancreatol 2:16–21
Liu Q, Liao Q, Zhao Y (2017) Chemotherapy and tumor microenvironment of pancreatic cancer. Cancer Cell Int 17:68
Neoptolemos JP, Palmer DH, Ghaneh P et al (2017) Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 389:1011–1024
Neesse A, Algul H, Tuveson DA et al (2015) Stromal biology and therapy in pancreatic cancer: a changing paradigm. Gut 64:1476–1484
Kleeff J, Korc M, Apte M et al (2016) Pancreatic cancer. Nat Rev Dis Primers 2:16022
Burkholder B, Huang RY, Burgess R et al (2014) Tumor-induced perturbations of cytokines and immune cell networks. Biochim Biophys Acta 1845:182–201
Liu Q, Li Y, Niu Z et al (2016) Atorvastatin (Lipitor) attenuates the effects of aspirin on pancreatic cancerogenesis and the chemotherapeutic efficacy of gemcitabine on pancreatic cancer by promoting M2 polarized tumor associated macrophages. J Exp Clin Cancer Res 35:33
Clark CE, Hingorani SR, Mick R et al (2007) Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res 67:9518–9527
Hou YC, Chao YJ, Hsieh MH et al (2019) Low CD8+ T cell infiltration and High PD-L1 expression are associated with level of CD44+/CD133+ cancer stem cells and predict an unfavorable prognosis in pancreatic cancer. Cancers (Basel) 11:E541
D'Costa Z, Jones K, Azad A et al (2017) Gemcitabine-induced TIMP1 attenuates therapy response and promotes tumor growth and liver metastasis in pancreatic cancer. Cancer Res 77:5952–5962
De Palma M, Lewis CE (2013) Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell 23:277–286
Noy R, Pollard JW (2014) Tumor-associated macrophages: from mechanisms to therapy. Immunity 41:49–61
Beatty GL, Chiorean EG, Fishman MP et al (2011) CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 331:1612–1616
Li C, Heidt DG, Dalerba P et al (2007) Identification of pancreatic cancer stem cells. Cancer Res 67:1030–1037
Baghdadi M, Wada H, Nakanishi S et al (2016) Chemotherapy-induced IL34 enhances immunosuppression by tumor-associated macrophages and mediates survival of chemoresistant lung cancer cells. Cancer Res 76:6030–6042
Gao Q, Qiu SJ, Fan J et al (2007) Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol 25:2586–2593
Chang W, Gao X, Han Y et al (2014) Gene expression profiling-derived immunohistochemistry signature with high prognostic value in colorectal carcinoma. Gut 63:1457–1467
Camp RL, Dolled-Filhart M, Rimm DL (2004) X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 10:7252–7259
The Lancet Gastroenterology H (2017) Pancreatic cancer: how can we tackle the lack of progress? Lancet Gastroenterol Hepatol 2:73
Ter Veer E, van Rijssen LB, Besselink MG et al (2018) Consensus statement on mandatory measurements in pancreatic cancer trials (COMM-PACT) for systemic treatment of unresectable disease. Lancet Oncol 19:e151–e160
Joyce JA, Fearon DT (2015) T cell exclusion, immune privilege, and the tumor microenvironment. Science 348:74–80
Bronte V, Murray PJ (2015) Understanding local macrophage phenotypes in disease: modulating macrophage function to treat cancer. Nat Med 21:117–119
Nielsen SR, Quaranta V, Linford A et al (2016) Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis. Nat Cell Biol 18:549–560
Mahajan UM, Langhoff E, Goni E et al (2018) Immune cell and stromal signature associated with progression-free survival of patients with resected pancreatic ductal adenocarcinoma. Gastroenterology 155:1625–1639
Posey AD Jr, Schwab RD, Boesteanu AC et al (2016) Engineered CAR T cells targeting the cancer-associated Tn-glycoform of the membrane mucin MUC1 control adenocarcinoma. Immunity 44:1444–1454
Topalian SL, Hodi FS, Brahmer JR et al (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443–2454
Ning P, Lou WH, Jun Y (2019) PD-1 immunotherapy in pancreatic cancer. J Panreatol 2:6–10
Gurlevik E, Fleischmann-Mundt B, Brooks J et al (2016) Administration of gemcitabine after pancreatic tumor resection in mice induces an antitumor immune response mediated by natural killer cells. Gastroenterology 151(338–350):e7
Hinz S, Pagerols-Raluy L, Oberg HH et al (2007) Foxp3 expression in pancreatic carcinoma cells as a novel mechanism of immune evasion in cancer. Cancer Res 67:8344–8350
Casella G, Garzetti L, Gatta AT et al (2016) IL4 induces IL6-producing M2 macrophages associated to inhibition of neuroinflammation in vitro and in vivo. J Neuroinflammation 13:139
Van Overmeire E, Stijlemans B, Heymann F et al (2016) M-CSF and GM-CSF receptor signaling differentially regulate monocyte maturation and macrophage polarization in the tumor microenvironment. Cancer Res 76:35–42
Ries CH, Cannarile MA, Hoves S et al (2014) Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell 25:846–859
Harris RJ, Pettitt AR, Schmutz C et al (2000) Granulocyte-macrophage colony-stimulating factor as an autocrine survival factor for mature normal and malignant B lymphocytes. J Immunol 164:3887–3893
Hong IS (2016) Stimulatory versus suppressive effects of GM-CSF on tumor progression in multiple cancer types. Exp Mol Med 48:e242
Bayne LJ, Beatty GL, Jhala N et al (2012) Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell 21:822–835
Neoptolemos JP, Kleeff J, Michl P et al (2018) Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol 15:333–348
Ahmed S, Bradshaw AD, Gera S et al (2017) The TGF-beta/Smad4 signaling pathway in pancreatic carcinogenesis and its clinical significance. J Clin Med 6:5
Abel EV, Simeone DM (2013) Biology and clinical applications of pancreatic cancer stem cells. Gastroenterology 144:1241–1248
Kreso A, Dick JE (2014) Evolution of the cancer stem cell model. Cell Stem Cell 14:275–291
Hermann PC, Huber SL, Herrler T et al (2007) Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 1:313–323
Riabov V, Gudima A, Wang N et al (2014) Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front Physiol 5:75
Schmidt A, Zhang XM, Joshi RN et al (2016) Human macrophages induce CD4(+)Foxp3(+) regulatory T cells via binding and re-release of TGF-beta. Immunol Cell Biol 94:747–762
Hughes R, Qian BZ, Rowan C et al (2015) Perivascular M2 macrophages stimulate tumor relapse after chemotherapy. Cancer Res 75:3479–3491
Poth KJ, Guminski AD, Thomas GP et al (2010) Cisplatin treatment induces a transient increase in tumorigenic potential associated with high interleukin-6 expression in head and neck squamous cell carcinoma. Mol Cancer Ther 9:2430–2439
Takeuchi S, Baghdadi M, Tsuchikawa T et al (2015) Chemotherapy-derived inflammatory responses accelerate the formation of immunosuppressive myeloid cells in the tissue microenvironment of human pancreatic cancer. Cancer Res 75:2629–2640
Acknowledgements
We sincerely expressed our thanks to Junyi Pang, Liangrui Zhou, Xiaolong Liang and Xuguang Liu for their excellent pathological work.
Funding
This work was supported by National Natural Science Foundation of China (81673023, 81872501, 81502068, 81272573 and 81560387) and Beijing Natural Science Foundation of China (7172177) and CAMS innovation Fun for Medical Sciences (CIFMS), NWA0564-3.
Author information
Authors and Affiliations
Contributions
QL, YZ, QL and ZL designed the study. QL, WW, YL, RZ, XG, MC, XZ, JL, TL and BP collected and analyzed the data. RZ, JL and TL performed the in vitro experiments. QL, QL, YZ and JK discussed and wrote the manuscript. WW, WW, JG, MD, TZ, QL and YZ performed the operations and analyzed the data. ZL, HW and YL performed the pathological work. JK, QL and YZ revised the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All of the authors declared there was no conflict of interest.
Ethical approval and ethical standards
This study was a retrospective study and was approved by the Ethics Committee of Peking Union Medical College Hospital and was registered at ClinicalTrials.org (NCT02654288).
Informed consent
All of the patients signed the informed consent to donate samples for scientific research. In this study, only resected tumor and peritumor sample were used; therefore, there were nearly no potential harms to the patients.
Human and animal rights
Male 4-week-old or 8-week-old C57BL/6 J mice were purchased from VITAL RIVER Animal Center (Beijing, China). Animal studies were performed in accordance with the institutional guidelines of Peking Union Medical College Hospital and was approved by animal study committee of Peking Union Medical College Hospital on December 1, 2014.
Cell line authentication
All cell lines were purchased from Cell Line Bank of Chinese Academy of Medicine Sciences.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, Q., Wu, H., Li, Y. et al. Combined blockade of TGf-β1 and GM-CSF improves chemotherapeutic effects for pancreatic cancer by modulating tumor microenvironment. Cancer Immunol Immunother 69, 1477–1492 (2020). https://doi.org/10.1007/s00262-020-02542-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02542-7