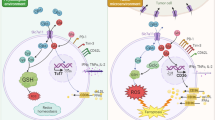
Abstract
Amino acid deprivation is a strategy that malignancies utilize to blunt anti-tumor T-cell immune responses. It has been proposed that amino acid insufficiency in T-cells is detected by GCN2 kinase, which through phosphorylation of EIF2α, shuts down global protein synthesis leading to T-cell arrest. The role of this amino acid stress sensor in the context of malignant brain tumors has not yet been studied, and may elucidate important insights into the mechanisms of T-cell survival in this harsh environment. Using animal models of glioblastoma and animals with deficiency in GCN2, we explored the importance of this pathway in T-cell function within brain tumors. Our results show that GCN2 deficiency limited CD8+ T-cell activation and expression of cytotoxic markers in two separate murine models of glioblastoma in vivo. Importantly, adoptive transfer of antigen-specific T-cells from GCN2 KO mice did not control tumor burden as well as wild-type CD8+ T-cells. Our in vitro and in vivo data demonstrated that reduction in amino acid availability caused GCN2 deficient CD8+ T-cells to become rapidly necrotic. Mechanistically, reduced CD8+ T-cell activation and necrosis was due to a disruption in TCR signaling, as we observed reductions in PKCθ and phoshpo-PKCθ on CD8+ T-cells from GCN2 KO mice in the absence of tryptophan. Validating these observations, treatment of wild-type CD8+ T-cells with a downstream inhibitor of GCN2 activation also triggered necrosis of CD8+ T-cells in the absence of tryptophan. In conclusion, our data demonstrate the vital importance of intact GCN2 signaling on CD8+ T-cell function and survival in glioblastoma.






Similar content being viewed by others
Abbreviations
- ATF4:
-
Activating transcription factor 4
- CCR7:
-
C–C Chemokine receptor type 7
- CNS:
-
Central nervous system
- EAE:
-
Experimental autoimmune encephalomyelitis
- EIF2α:
-
Eukaryotic translation initiation factor 2α
- ER:
-
Endoplasmic reticular
- GCN2:
-
General control nonderepressible 2
- GZMB:
-
Granzyme B
- i.c.:
-
Intracranial
- ISRIB:
-
Integrated stress response inhibitor
- ISR:
-
Integrated stress response
- KO:
-
Knockout
- KYN:
-
Kynurenine
- LC-MS:
-
Liquid chromatography–mass spectrometry
- NCI:
-
National Cancer Institute
- PKC-ϴ:
-
Protein kinase C-theta
- + TRP:
-
Tryptophan added
- − TRP:
-
Tryptophan free
- WT:
-
Wild type
References
Rashidi A, Miska J, Pituch K, Kanojia D, Lopez-Rosas A, Han Y et al (2017) Gcn2 kinase is essential for adaptive T-cell immunity in glioma. Neuro-Oncology 19:113
Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM et al (2013) Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 369(2):122–133
Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M et al (2018) Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol 36(8):773–779
Cloughesy TF, Mochizuki AY, Orpilla JR, Hugo W, Lee AH, Davidson TB et al (2019) Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med 25(3):477–486
Woroniecka K, Chongsathidkiet P, Rhodin K, Kemeny H, Dechant C, Farber SH et al (2018) T-cell exhaustion signatures vary with tumor type and are severe in glioblastoma. Clin Cancer Res 24(17):4175–4186
Mirzaei R, Sarkar S, Yong VW (2017) T cell exhaustion in glioblastoma: intricacies of immune checkpoints. Trends Immunol 38(2):104–115
Wei J, Raynor J, Nguyen TL, Chi H (2017) Nutrient and metabolic sensing in T cell responses. Front Immunol 8:247
Kwidzinski E, Bunse J, Aktas O, Richter D, Mutlu L, Zipp F et al (2005) Indolamine 2,3-dioxygenase is expressed in the CNS and down-regulates autoimmune inflammation. Faseb J. 19(8):1347
Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B et al (1998) Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 281(5380):1191–1193
Lob S, Konigsrainer A, Zieker D, Brucher BL, Rammensee HG, Opelz G et al (2009) IDO1 and IDO2 are expressed in human tumors: levo—but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunol Immunother 58(1):153–157
Dong J, Qiu H, Garcia-Barrio M, Anderson J, Hinnebusch AG (2000) Uncharged tRNA activates GCN2 by displacing the protein kinase moiety from a bipartite tRNA-binding domain. Mol Cell 6(2):269–279
Garcia-Barrio M, Dong J, Ufano S, Hinnebusch AG (2000) Association of GCN1-GCN20 regulatory complex with the N-terminus of eIF2alpha kinase GCN2 is required for GCN2 activation. EMBO J 19(8):1887–1899
Fusakio ME, Willy JA, Wang Y, Mirek ET, Al Baghdadi RJ, Adams CM et al (2016) Transcription factor ATF4 directs basal and stress-induced gene expression in the unfolded protein response and cholesterol metabolism in the liver. Mol Biol Cell 27(9):1536–1551
Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M et al (2000) Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell 6(5):1099–1108
Pakos-Zebrucka K, Koryga I, Mnich K, Ljujic M, Samali A, Gorman AM (2016) The integrated stress response. EMBO Rep 17(10):1374–1395
Wainwright DA, Balyasnikova IV, Chang AL, Ahmed AU, Moon KS, Auffinger B et al (2012) IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin Cancer Res 18(22):6110–6121
Wainwright DA, Chang AL, Dey M, Balyasnikova IV, Kim C, Tobias AL et al (2014) Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4 and PD-L1 in mice with brain tumors. Clinical Cancer Research. 20:5290–5301
Holmgaard RB, Zamarin D, Li YY, Gasmi B, Munn DH, Allison JP et al (2015) Tumor-expressed IDO recruits and activates MDSCs in a treg-dependent manner. Cell Rep 13(2):412–424
Yu JP, Du WJ, Yan F, Wang Y, Li H, Cao S et al (2013) Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol 190(7):3783–3797
Miret JJ, Kirschmeier P, Koyama S, Zhu M, Li YY, Naito Y et al (2019) Suppression of myeloid cell arginase activity leads to therapeutic response in a NSCLC mouse model by activating anti-tumor immunity. J Immunother Cancer 7(1):32
Lind DS (2004) Arginine and cancer. J Nutr. 134(10 Suppl):2837S–2841S (discussion 53S)
Caso G, McNurlan MA, McMillan ND, Eremin O, Garlick PJ (2004) Tumour cell growth in culture: dependence on arginine. Clin Sci (Lond) 107(4):371–379
Geiger R, Rieckmann JC, Wolf T, Basso C, Feng Y, Fuhrer T et al (2016) l-Arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell. 167(3):829–842.e13
Zhang J, Fan J, Venneti S, Cross JR, Takagi T, Bhinder B et al (2014) Asparagine plays a critical role in regulating cellular adaptation to glutamine depletion. Mol Cell 56(2):205–218
Pavlova NN, Hui S, Ghergurovich JM, Fan J, Intlekofer AM, White RM et al (2018) As extracellular glutamine levels decline, asparagine becomes an essential amino acid. Cell Metabolism. 27(2):428
Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D et al (2005) GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity 22(5):633–642
Van de Velde LA, Guo XJ, Barbaric L, Smith AM, Oguin TH 3rd, Thomas PG et al (2016) Stress kinase GCN2 controls the proliferative fitness and trafficking of cytotoxic T cells independent of environmental amino acid sensing. Cell Rep 17(9):2247–2258
Keil M, Sonner JK, Lanz TV, Oezen I, Bunse T, Bittner S et al (2016) General control non-derepressible 2 (GCN2) in T cells controls disease progression of autoimmune neuroinflammation. J Neuroimmunol 297:117–126
Nakamura A, Nambu T, Ebara S, Hasegawa Y, Toyoshima K, Tsuchiya Y et al (2018) Inhibition of GCN2 sensitizes ASNS-low cancer cells to asparaginase by disrupting the amino acid response. Proc Natl Acad Sci USA 115(33):E7776–E7785
Nguyen HG, Conn CS, Kye Y, Xue LR, Forester CM, Cowan JE et al (2018) Development of a stress response therapy targeting aggressive prostate cancer. Sci Trans Med 10(439):eaar2036
Kim JW, Miska J, Young JS, Rashidi A, Kane JR, Panek WK et al (2017) A comparative study of replication-incompetent and -competent adenoviral therapy-mediated immune response in a murine glioma model. Mol Ther Oncolytics 5:97–104
Wu YM, Li L (2016) Sample normalization methods in quantitative metabolomics. J Chromatogr A 1430:80–95
Clarke SRM, Barnden M, Kurts C, Carbone FR, Miller JF, Heath WR (2000) Characterization of the ovalbumin-specific TCR transgenic line OT-I: MHC elements for positive and negative selection. Immunol Cell Biol 78(2):110–117
Bjorkdahl O, Barber KA, Brett SJ, Daly MG, Plumpton C, Elshourbagy NA et al (2003) Characterization of CC-chemokine receptor 7 expression on murine T cells in lymphoid tissues. Immunology 110(2):170–179
Ladomersky E, Zhai L, Lenzen A, Lauing KL, Qian J, Scholtens DM et al (2018) IDO1 inhibition synergizes with radiation and PD-1 blockade to durably increase survival against advanced glioblastoma. Clin Cancer Res 24(11):2559–2573
Sonner JK, Deumelandt K, Ott M, Thome CM, Rauschenbach KJ, Schulz S et al (2016) The stress kinase GCN2 does not mediate suppression of antitumor T cell responses by tryptophan catabolism in experimental melanomas. Oncoimmunology 5(12):e1240858
Hayashi K, Altman A (2007) Protein kinase C theta (PKCtheta): a key player in T cell life and death. Pharmacol Res 55(6):537–544
Sidrauski C, McGeachy AM, Ingolia NT, Walter P (2015) The small molecule ISRIB reverses the effects of eIF2alpha phosphorylation on translation and stress granule assembly. Elife. https://doi.org/10.7554/eLife.05033
Behrens PF, Langemann H, Strohschein R, Draeger J, Hennig J (2000) Extracellular glutamate and other metabolites in and around RG2 rat glioma: an intracerebral microdialysis study. J Neurooncol 47(1):11–22
Wibom C, Surowiec I, Moren L, Bergstrom P, Johansson M, Antti H et al (2010) Metabolomic patterns in glioblastoma and changes during radiotherapy: a clinical microdialysis study. J Proteome Res 9(6):2909–2919
Ho PC, Bihuniak JD, Macintyre AN, Staron M, Liu X, Amezquita R et al (2015) Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell 162(6):1217–1228
Chang CH, Qiu J, O’Sullivan D, Buck MD, Noguchi T, Curtis JD et al (2015) Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162(6):1229–1241
Hayashi K, Jutabha P, Endou H, Sagara H, Anzai N (2013) LAT1 is a critical transporter of essential amino acids for immune reactions in activated human T cells. J Immunol 191(8):4080–4085
Nawashiro H, Otani N, Uozumi Y, Ooigawa H, Toyooka T, Suzuki T et al (2005) High expression of L-type amino acid transporter 1 in infiltrating glioma cells. Brain Tumor Pathol 22(2):89–91
Kobayashi K, Ohnishi A, Promsuk J, Shimizu S, Kanai Y, Shiokawa Y et al (2008) Enhanced tumor growth elicited by L-type amino acid transporter 1 in human malignant glioma cells. Neurosurgery. 62(2):493–503 (discussion -4)
Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Yang THO et al (2018) The immune landscape of cancer. Immunity. 48(4):812
Hendry S, Salgado R, Gevaert T, Russell PA, John T, Thapa B et al (2017) Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the international immuno-oncology biomarkers working group: part 2: TILs in melanoma, gastrointestinal tract carcinomas, non-small cell lung carcinoma and mesothelioma, endometrial and ovarian carcinomas, squamous cell carcinoma of the head and neck, genitourinary carcinomas, and primary brain tumors. Adv Anat Pathol 24(6):311–335
Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J et al (2013) Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys 86(2):343–349
Kleijn A, van den Bossche W, Haefner ES, Belcaid Z, Burghoorn-Maas C, Kloezeman JJ et al (2017) The sequence of Delta24-RGD and TMZ administration in malignant glioma affects the role of CD8(+)T cell anti-tumor activity. Mol Ther Oncolytics 5:11–19
Cao Y, Rathmell JC, Macintyre AN (2014) Metabolic reprogramming towards aerobic glycolysis correlates with greater proliferative ability and resistance to metabolic inhibition in CD8 versus CD4 T cells. PLoS One 9(8):e104104
Chongsathidkiet P, Jackson C, Koyama S, Loebel F, Cui X, Farber SH et al (2018) Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med 24(9):1459–1468
Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL (1999) Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med 189(9):1363–1372
Prendergast GC, Malachowski WP, DuHadaway JB, Muller AJ (2017) Discovery of IDO1 inhibitors: from bench to bedside. Cancer Res 77(24):6795–6811
Galezowski M, Sitarz K, Majewska E, Chmielewski S, Michalik K, Masiejczyk M et al (2017) Development of small molecule selective inhibitors of GCN2 as an immunotherapy aimed at preventing immune escape of tumor cells. Cancer Res 77:2639
Wek RC, Jiang HY, Anthony TG (2006) Coping with stress: eIF2 kinases and translational control. Biochem Soc T 34:7–11
B’chir W, Maurin AC, Carraro V, Averous J, Jousse C, Muranishi Y et al (2013) The eIF2 alpha/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res. 41(16):7683–7699
Xu XJ, Araki K, Li SZ, Han JH, Ye LL, Tan WG et al (2014) Autophagy is essential for effector CD8(+) T cell survival and memory formation. Nat Immunol 15(12):1152–1161
Wilson GJ, Bunpo P, Cundiff JK, Wek RC, Anthony TG (2013) The eukaryotic initiation factor 2 kinase GCN2 protects against hepatotoxicity during asparaginase treatment. Am J Physiol Endocrinol Metab 305(9):E1124–E1133
She P, Bunpo P, Cundiff JK, Wek RC, Harris RA, Anthony TG (2013) General control nonderepressible 2 (GCN2) kinase protects oligodendrocytes and white matter during branched-chain amino acid deficiency in mice. J Biol Chem 288(43):31250–31260
Chaudhary K, Shinde R, Liu HY, Gnana-Prakasam JP, Veeranan-Karmegam R, Huang L et al (2015) Amino acid metabolism inhibits antibody-driven kidney injury by inducing autophagy. J Immunol 194(12):5713–5724
Xu Y, Poggio M, Jin HY, Shi Z, Forester CM, Wang Y et al (2019) Translation control of the immune checkpoint in cancer and its therapeutic targeting. Nat Med 25(2):301–311
Acknowledgements
We would like to thank Peng Gao at the Metabolomics Core Facility at Northwestern for performing and analyzing bulk metabolomics included in this study.
Funding
Financial support comes from an Outstanding Investigator Award from the National Cancer Institute (NCI) to Maciej S Lesniak (R35CA197725) and a grant from the National Institute of Neurological Disorders and Stroke (NINDS) to Maciej S Lesniak (R01NS093903). Jason Miska received a fellowship from the NINDS (1F32NS098737-01A1). This work was supported by the Northwestern University Robert H. Lurie Cancer Center Flow Cytometry Facility Support Grant (NCI CA060553).
Author information
Authors and Affiliations
Contributions
AR, JM, and MSL conceived the study. AR, JM, CLC, PZ, DK, and JWK designed and performed the experiments. ALR maintained animal colonies, performed and analyzed the GCN2 backcrosses, and validated all mice used in this study via genotyping. YH and WKP performed all animal surgeries and monitoring of endpoint analyses. KCP, JF, and MT assisted with the construction and provided critical feedback for the manuscript. MSL and JM oversaw the research program and assisted in the preparation of this manuscript. TX performed all statistical analyses and provided feedback regarding animal numbers. All authors have reviewed the manuscript for accuracy and provided feedback during the writing and revision process.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and ethical standards
The IACUC committee approved all animal work within the Center for Comparative Medicine (CCM) at Northwestern University. Northwestern University has an Animal Welfare Assurance on file with the Office of Laboratory Animal Welfare (A3283-01). Northwestern University conducts its reviews following United States Public Health Service (USPHS) regulations and applicable federal and local laws. The composition of the IACUC meets the requirements of the USPHS policy and the Animal Welfare Act Regulations. The animal protocol number is IS00002459.
Animal source
C57BL/6 (WT), GCN2 KO, Rag1 KO, OT-1, and Foxp3-IRES-GFP C57/B6 mice were purchased directly from The Jackson Laboratory (Bar Harbor, ME).
Cell line authentication
GL-261 was purchased directly from the National Cancer Institute (NCI) Frederick National Tumor Repository Laboratory (Frederick, MD. USA). CT2A was acquired from the Balyasnikova laboratory at Northwestern University, Feinberg School of Medicine (Chicago, IL. USA). All cell lines were used in Northwestern’s Neuro-oncology research. All cell lines are routinely tested for Mycoplasma contamination every 2 months using the Universal Mycoplasma Detection Kit (ATCC® 30-1012 K™). The identity and purity of cell lines were determined using short tandem repeats (STR) profiling performed by the Northwestern sequencing facility.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rashidi, A., Miska, J., Lee-Chang, C. et al. GCN2 is essential for CD8+ T cell survival and function in murine models of malignant glioma. Cancer Immunol Immunother 69, 81–94 (2020). https://doi.org/10.1007/s00262-019-02441-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-019-02441-6