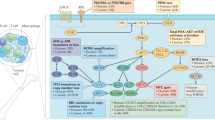
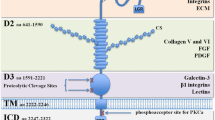
Abstract
Despite the significant progress in tumor prevention, early detection, diagnosis and treatment made over recent decades, cancer is still an enormous public health challenge all around the world, with the number of people affected increasing every year. A great deal of effort is therefore being devoted to the search for novel safe, effective and economically sustainable treatments for the growing population of neoplastic patients. One main obstacle to this process is the extremely low percentage of therapeutic approaches that, after successfully passing pre-clinical testing, actually demonstrate activity when finally tested in humans. This disappointing and expensive failure rate is partly due to the pre-clinical murine models used for in vivo testing, which cannot faithfully recapitulate the multifaceted nature and evolution of human malignancies. These features are better mirrored in natural disease models, i.e., companion animals affected by cancers. Herein, we discuss the relevance of spontaneous canine tumors for the evaluation of the safety and anti-tumor activity of novel therapeutic strategies before in-human trials, and present our experience in the development of a vaccine that targets chondroitin sulphate proteoglycan (CSPG)4 as an example of these comparative oncology studies.




Similar content being viewed by others
Abbreviations
- BTK:
-
Bruton’s tyrosine kinase
- CAR-T:
-
Genetically engineered T cells with chimeric antigen receptors
- CSC:
-
Cancer stem cells
- CIs:
-
Checkpoint inhibitors
- CSPG4:
-
Chondroitin sulphate proteoglycan 4
- COTC:
-
Comparative Oncology Trials Consortium
- CTLA-4:
-
Cytotoxic T lymphocyte antigen-4
- ECM:
-
Extracellular matrix
- FAK:
-
Focal adhesion kinase
- FDA:
-
Food and Drug Administration
- IHC:
-
Immunohistochemical
- L-MTP-PE:
-
Liposomal muramyl tripeptide phosphatidyl ethanolamine
- MM:
-
Malignant melanoma
- NCI:
-
National Cancer Institute
- OSA:
-
Osteosarcoma
- PDX:
-
Patients-derived xenograft
- PAC-1:
-
Procaspase-activating compound-1
- PD-1:
-
Programmed cell death receptor-1
- TWT:
-
Triple wild type
- USDA:
-
United States Department of Agriculture
- WT:
-
Wild type
References
Dragani TA, Castells A, Kulasingam V, Diamandis EP, Earl H, Iams WT, Lovly CM, Sedelaar JP, Schalken JA (2016) Major milestones in translational oncology. BMC Med 14:110. https://doi.org/10.1186/s12916-016-0654-y
Barutello G, Rolih V, Arigoni M, Tarone L, Conti L, Quaglino E, Buracco P, Cavallo F, Riccardo F (2018) Strengths and weaknesses of pre-clinical models for human melanoma treatment: dawn of dogs’ revolution for immunotherapy. Int J Mol Sci. https://doi.org/10.3390/ijms19030799
Mak IW, Evaniew N, Ghert M (2014) Lost in translation: animal models and clinical trials in cancer treatment. Am J Transl Res 6:114–118
Riccardo F, Aurisicchio L, Impellizeri JA, Cavallo F (2015) The importance of comparative oncology in translational medicine. Cancer Immunol Immunother CII 64:137–148. https://doi.org/10.1007/s00262-014-1645-5
Paoloni M, Khanna C (2008) Translation of new cancer treatments from pet dogs to humans. Nat Rev Cancer 8:147–156. https://doi.org/10.1038/nrc2273
Talmadge JE, Singh RK, Fidler IJ, Raz A (2007) Murine models to evaluate novel and conventional therapeutic strategies for cancer. Am J Pathol 170:793–804. https://doi.org/10.2353/ajpath.2007.060929
Gordon MY, Blackett NM (1976) The sensitivities of human and murine hemopoietic cells exposed to cytotoxic drugs in an in vivo culture. Cancer Res 36:2822–2826
Honigberg LA, Smith AM, Sirisawad M et al (2010) The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A. 107:13075–13080. https://doi.org/10.1073/pnas.1004594107
Krogh A (1929) The progress of physiology. Science 70:200–204. https://doi.org/10.1126/science.70.1809.200
Lindblad-Toh K, Wade CM, Mikkelsen TS et al (2005) Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature 438:803–819. https://doi.org/10.1038/nature04338
Olson PN (2007) Using the canine genome to cure cancer and other diseases. Theriogenology 68:378–381. https://doi.org/10.1016/j.theriogenology.2007.04.016
Mueller F, Fuchs B, Kaser-Hotz B (2007) Comparative biology of human and canine osteosarcoma. Anticancer Res 27:155–164
Gardner HL, Fenger JM, London CA (2016) Dogs as a model for cancer. Annu Rev Anim Biosci 4:199–222. https://doi.org/10.1146/annurev-animal-022114-110911
Medicine Io, National Academies of Sciences E, Medicine (2015) The role of clinical studies for pets with naturally occurring tumors in translational cancer research: workshop summary. The National Academies Press, Washington, DC
Abdelmegeed SM, Mohammed S (2018) Canine mammary tumors as a model for human disease. Oncol Lett 15:8195–8205. https://doi.org/10.3892/ol.2018.8411
Fan TM, Selting KA (2018) Exploring the potential utility of pet dogs with cancer for studying radiation-induced immunogenic cell death strategies. Front Oncol 8:680. https://doi.org/10.3389/fonc.2018.00680
Hay M, Thomas DW, Craighead JL, Economides C, Rosenthal J (2014) Clinical development success rates for investigational drugs. Nat Biotechnol 32:40–51. https://doi.org/10.1038/nbt.2786
Barsoum N, Kleeman C (2002) Now and then, the history of parenteral fluid administration. Am J Nephrol 22:284–289. https://doi.org/10.1159/000063775
Murray JE, Sheil AG, Moseley R, Knight P, McGavic JD, Dammin GJ (1964) Analysis of mechanism of immunosuppressive drugs in renal homotransplantation. Ann Surg 160:449–473
Weiden PL, Storb R, Lerner KG, Kao GF, Graham TC, Thomas ED (1975) Treatment of canine malignancies by 1200 R total body irradiation and autologous marrow grafts. Exp Hematol 3:124–134
Storb R, Tsoi MS, Weiden PL, Graham TC, Thomas ED (1976) Studies on the mechanism of stable graft-host tolerance in canine and human radiation chimeras. Transplant Proc 8:561–564
Faivre S, Delbaldo C, Vera K et al (2006) Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol 24:25–35. https://doi.org/10.1200/JCO.2005.02.2194
London CA, Bernabe LF, Barnard S et al (2014) Preclinical evaluation of the novel, orally bioavailable selective inhibitor of nuclear export (SINE) KPT-335 in spontaneous canine cancer: results of a phase I study. PLoS ONE 9:e87585. https://doi.org/10.1371/journal.pone.0087585
Burger JA, Keating MJ, Wierda WG et al (2014) Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: a single-arm, phase 2 study. Lancet Oncol 15:1090–1099. https://doi.org/10.1016/s1470-2045(14)70335-3
Vail DM, Thamm DH, Reiser H et al (2009) Assessment of GS-9219 in a pet dog model of non-Hodgkin’s lymphoma. Clin Cancer Res 15:3503–3510. https://doi.org/10.1158/1078-0432.CCR-08-3113
Reiser H, Wang J, Chong L et al (2008) GS-9219–a novel acyclic nucleotide analogue with potent antineoplastic activity in dogs with spontaneous non-Hodgkin’s lymphoma. Clin Cancer Res 14:2824–2832. https://doi.org/10.1158/1078-0432.CCR-07-2061
De Clercq E (2018) Tanovea(R) for the treatment of lymphoma in dogs. Biochem Pharmacol 154:265–269. https://doi.org/10.1016/j.bcp.2018.05.010
West DC, Qin Y, Peterson QP et al (2012) Differential effects of procaspase-3 activating compounds in the induction of cancer cell death. Mol Pharm 9:1425–1434. https://doi.org/10.1021/mp200673n
Peterson QP, Hsu DC, Novotny CJ, West DC, Kim D, Schmit JM, Dirikolu L, Hergenrother PJ, Fan TM (2010) Discovery and canine preclinical assessment of a nontoxic procaspase-3-activating compound. Cancer Res 70:7232–7241. https://doi.org/10.1158/0008-5472.CAN-10-0766
Joshi AD, Botham RC, Schlein LJ et al (2017) Synergistic and targeted therapy with a procaspase-3 activator and temozolomide extends survival in glioma rodent models and is feasible for the treatment of canine malignant glioma patients. Oncotarget. https://doi.org/10.18632/oncotarget.19085
Bergman PJ, Camps-Palau MA, McKnight JA et al (2006) Development of a xenogeneic DNA vaccine program for canine malignant melanoma at the animal medical center. Vaccine 24:4582–4585. https://doi.org/10.1016/j.vaccine.2005.08.027
Liao JC, Gregor P, Wolchok JD, Orlandi F, Craft D, Leung C, Houghton AN, Bergmann PJ (2006) Vaccination with human tyrosinase DNA induces antibody responses in dogs with advanced melanoma. Cancer Immun 6:21
Grosenbaugh DA, Leard AT, Bergman PJ et al (2011) Safety and efficacy of a xenogeneic DNA vaccine encoding for human tyrosinase as adjunctive treatment for oral malignant melanoma in dogs following surgical excision of the primary tumor. Am J Vet Res. https://doi.org/10.2460/ajvr.72.12.1631
Wolchok JD, Yuan J, Houghton AN et al (2007) Safety and immunogenicity of tyrosinase DNA vaccines in patients with melanoma. Molecular Ther J Am Soc Gene Ther 15:2044–2050. https://doi.org/10.1038/sj.mt.6300290
Yuan J, Ku GY, Adamow M et al (2013) Immunologic responses to xenogeneic tyrosinase DNA vaccine administered by electroporation in patients with malignant melanoma. J Immunother Cancer. https://doi.org/10.1186/2051-1426-1-20
Ottnod JM, Smedley RC, Walshaw R, Hauptman JG, Kiupel M, Obradovich JE (2013) A retrospective analysis of the efficacy of Oncept vaccine for the adjunct treatment of canine oral malignant melanoma. Vet Comp Oncol 11:219–229. https://doi.org/10.1111/vco.12057
Treggiari E, Grant JP, North SM (2016) A retrospective review of outcome and survival following surgery and adjuvant xenogeneic DNA vaccination in 32 dogs with oral malignant melanoma. J Vet Med Sci 78:845–850. https://doi.org/10.1292/jvms.15-0510
Verganti S, Berlato D, Blackwood L, Amores-Fuster I, Polton GA, Elders R, Doyle R, Taylor A, Murphy S (2017) Use of Oncept melanoma vaccine in 69 canine oral malignant melanomas in the UK. J Small Anim Pract 58:10–16. https://doi.org/10.1111/jsap.12613
Piras LA, Riccardo F, Iussich S et al (2017) Prolongation of survival of dogs with oral malignant melanoma treated by en bloc surgical resection and adjuvant CSPG4-antigen electrovaccination. Vet Comp Oncol 15:996–1013. https://doi.org/10.1111/vco.12239
Riccardo F, Iussich S, Maniscalco L et al (2014) CSPG4-specific immunity and survival prolongation in dogs with oral malignant melanoma immunized with human CSPG4 DNA. Clin Cancer Res Off J Am Assoc Cancer Res 20:3753–3762. https://doi.org/10.1158/1078-0432.CCR-13-3042
Kurzman ID, MacEwen EG, Rosenthal RC et al (1995) Adjuvant therapy for osteosarcoma in dogs—results of randomized clinical trials using combined liposome-encapsulated muramyl tripeptide and cisplatin. Clin Cancer Res 1:1595–1601
Kleinerman ES, Gano JB, Johnston DA, Benjamin RS, Jaffe N (1995) Efficacy of liposomal muramyl tripeptide (CGP 19835A) in the treatment of relapsed osteosarcoma. Am J Clin Oncol 18:93–99
Kleinerman ES, Jia SF, Griffin J, Seibel NL, Benjamin RS, Jaffe N (1992) Phase II study of liposomal muramyl tripeptide in osteosarcoma: the cytokine cascade and monocyte activation following administration. J Clin Oncol 10:1310–1316
Creaven PJ, Cowens JW, Brenner DE et al (1990) Initial clinical trial of the macrophage activator MTP-PE encapsulated in liposomes in patients with advanced cancer. J Biol Resp Modifier 9:492–498
Pa M (2009) Muramyl tripeptide (mifamurtide) for the treatment of osteosarcoma. Expert Rev Anticancer Ther. https://doi.org/10.1586/era.09.69
Biteau K, Guiho R, Chatelais M, Taurelle J, Chesneau J, Corradini N, Heymann D, Redini F (2016) L-MTP-PE and zoledronic acid combination in osteosarcoma- preclinical evidence of positive therapeutic combination for clinical transfer. Am J Cancer Res 6:677
Mason NJ, Gnanandarajah JS, Engiles JB, Gray F, Laughlin D, Gaurnier-Hausser A, Wallecha A, Huebner M, Paterson Y (2016) Immunotherapy with a HER2-Targeting Listeria Induces HER2-Specific Immunity and Demonstrates Potential Therapeutic Effects in a Phase I Trial in Canine Osteosarcoma. Clin Cancer Res Off J Am Assoc Cancer Res 22:4380–4390. https://doi.org/10.1158/1078-0432.CCR-16-0088
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68:7–30. https://doi.org/10.3322/caac.21442
Jenkins RW, Barbie DA, Flaherty KT (2018) Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer 118:9–16. https://doi.org/10.1038/bjc.2017.434
Nishiya AT, Massoco CO, Felizzola CR et al (2016) Comparative Aspects of Canine Melanoma. Vet Sci. https://doi.org/10.3390/vetsci3010007
Bosenberg M, Arnheiter H, Kelsh R (2014) Melanoma in mankind’s best friend. Pigment Cell Melanoma Res 27:1. https://doi.org/10.1111/pcmr.12196
Mochizuki H, Kennedy K, Shapiro SG, Breen M (2015) BRAF mutations in canine cancers. PLoS ONE 10:e0129534. https://doi.org/10.1371/journal.pone.0129534
Taran SJ, Taran R, Malipatil NB (2017) Pediatric osteosarcoma: an updated review. Indian J Med Paediatr Oncol 38:33–43. https://doi.org/10.4103/0971-5851.203513
Varshney J, Scott MC, Largaespada DA, Subramanian S (2016) Understanding the osteosarcoma pathobiology: a comparative oncology approach. Vet Sci. https://doi.org/10.3390/vetsci3010003
Fenger JM, London CA, Kisseberth WC (2014) Canine osteosarcoma: a naturally occurring disease to inform pediatric oncology. ILAR J 55:69–85. https://doi.org/10.1093/ilar/ilu009
Nicolosi PA, Dallatomasina A, Perris R (2015) Theranostic impact of NG2/CSPG4 proteoglycan in cancer. Theranostics 5:530–544. https://doi.org/10.7150/thno.10824
Benassi MS, Pazzaglia L, Chiechi A, Alberghini M, Conti A, Cattaruzza S, Wassermann B, Picci P, Perris R (2009) NG2 expression predicts the metastasis formation in soft-tissue sarcoma patients. J Orthop Res Off Publ Orthop Res Soc 27:135–140. https://doi.org/10.1002/jor.20694
Wang X, Katayama A, Wang Y et al (2011) Functional characterization of an scFv-Fc antibody that immunotherapeutically targets the common cancer cell surface proteoglycan CSPG4. Cancer Res 71:7410–7422. https://doi.org/10.1158/0008-5472.CAN-10-1134
Garusi E, Rossi S, Perris R (2012) Antithetic roles of proteoglycans in cancer. Cell Mol Life Sci CMLS 69:553–579. https://doi.org/10.1007/s00018-011-0816-1
Beard RE, Abate-Daga D, Rosati SF, Zheng Z, Wunderlich JR, Rosenberg SA, Morgan RA (2013) Gene expression profiling using nanostring digital RNA counting to identify potential target antigens for melanoma immunotherapy. Clinical Cancer Res Off J Am Assoc Cancer Res 19:4941–4950. https://doi.org/10.1158/1078-0432.CCR-13-1253
Ziai MR, Imberti L, Nicotra MR, Badaracco G, Segatto O, Natali PG, Ferrone S (1987) Analysis with monoclonal antibodies of the molecular and cellular heterogeneity of human high molecular weight melanoma associated antigen. Cancer Res 47:2474–2480
Rivera Z, Ferrone S, Wang X, Jube S, Yang H, Pass HI, Kanodia S, Gaudino G, Carbone M (2012) CSPG4 as a target of antibody-based immunotherapy for malignant mesothelioma. Clin Cancer Res Off J Am Assoc Cancer Res 18:5352–5363. https://doi.org/10.1158/1078-0432.CCR-12-0628
Kozanoglu I, Boga C, Ozdogu H, Sozer O, Maytalman E, Yazici AC, Sahin FI (2009) Human bone marrow mesenchymal cells express NG2: possible increase in discriminative ability of flow cytometry during mesenchymal stromal cell identification. Cytotherapy 11:527–533. https://doi.org/10.1080/14653240902923153
Campoli M, Ferrone S, Wang X (2010) Functional and clinical relevance of chondroitin sulfate proteoglycan 4. Adv Cancer Res 109:73–121. https://doi.org/10.1016/B978-0-12-380890-5.00003-X
Cavallo F, De Giovanni C, Nanni P, Forni G, Lollini PL (2011) 2011: the immune hallmarks of cancer. Cancer Immunol Immunother 60:319–326. https://doi.org/10.1007/s00262-010-0968-0
Rolih V, Barutello G, Iussich S, De Maria R, Quaglino E, Buracco P, Cavallo F, Riccardo F (2017) CSPG4: a prototype oncoantigen for translational immunotherapy studies. J Transl Med 15:151. https://doi.org/10.1186/s12967-017-1250-4
Cattaruzza S, Ozerdem U, Denzel M et al (2013) Multivalent proteoglycan modulation of FGF mitogenic responses in perivascular cells. Angiogenesis 16:309–327. https://doi.org/10.1007/s10456-012-9316-7
Price MA, Colvin Wanshura LE, Yang J et al (2011) CSPG4, a potential therapeutic target, facilitates malignant progression of melanoma. Pigment Cell Melanoma Res 24:1148–1157. https://doi.org/10.1111/j.1755-148X.2011.00929.x
Goretzki L, Lombardo CR, Stallcup WB (2000) Binding of the NG2 proteoglycan to kringle domains modulates the functional properties of angiostatin and plasmin(ogen). J Biol Chem 275:28625–28633. https://doi.org/10.1074/jbc.M002290200
Tamburini E, Dallatomasina A, Quartararo J, Cortelazzi B, Mangieri D, Lazzaretti M, Perris R (2019) Structural deciphering of the NG2/CSPG4 proteoglycan multifunctionality. FASEB J Off Publ Fed Am Soc Exp Biol 33:3112–3128. https://doi.org/10.1096/fj.201801670R
Tamburini E, Dallatomasina A, Quartararo J, Cortelazzi B, Mangieri D, Lazzaretti M, Perris R (2018) Structural deciphering of the NG2/CSPG4 proteoglycan multifunctionality. FASEB J. fj201801670R. https://doi.org/10.1096/fj.201801670r
Campoli MR, Chang CC, Kageshita T, Wang X, McCarthy JB, Ferrone S (2004) Human high molecular weight-melanoma-associated antigen (HMW-MAA): a melanoma cell surface chondroitin sulfate proteoglycan (MSCP) with biological and clinical significance. Crit Rev Immunol 24:267–296
Cirenajwis H, Ekedahl H, Lauss M et al (2015) Molecular stratification of metastatic melanoma using gene expression profiling: prediction of survival outcome and benefit from molecular targeted therapy. Oncotarget 6:12297–12309. https://doi.org/10.18632/oncotarget.3655
Bogunovic D, O’Neill DW, Belitskaya-Levy I et al (2009) Immune profile and mitotic index of metastatic melanoma lesions enhance clinical staging in predicting patient survival. Proc Natl Acad Sci U S A 106:20429–20434. https://doi.org/10.1073/pnas.0905139106
Mayayo SL, Prestigio S, Maniscalco L et al (2011) Chondroitin sulfate proteoglycan-4: a biomarker and a potential immunotherapeutic target for canine malignant melanoma. Vet J 190:e26–e30. https://doi.org/10.1016/j.tvjl.2011.02.020
Riccardo F, Tarone L, Iussich S, Giacobino D, Arigoni M, Sammartano F, Morello E, Martano M, Gattino F, De Maria R, Ferrone S, Buracco P, Cavallo F (2019) Identification of CSPG4 as a promising target for translational combinatorial approaches in osteosarcoma. Ther Adv Med Oncol. https://doi.org/10.1177/1758835919855491
Ruiu R, Rolih V, Bolli E et al (2019) Fighting breast cancer stem cells through the immune-targeting of the xCT cystine-glutamate antiporter. Cancer Immunol Immunother CII 68:131–141. https://doi.org/10.1007/s00262-018-2185-1
Koren A, Rijavec M, Kern I, Sodja E, Korosec P, Cufer T (2016) BMI1, ALDH1A1, and CD133 transcripts connect epithelial-mesenchymal transition to cancer stem cells in lung carcinoma. Stem Cells Int 2016:9714315. https://doi.org/10.1155/2016/9714315
Rivera Z, Ferrone S, Wang X, Jube S, Yang H, Pass HI, Kanodia S, Gaudino G, Carbone M (2012) CSPG4 as a target of antibody-based immunotherapy for malignant mesothelioma. Clin Cancer Res 18:5352–5363. https://doi.org/10.1158/1078-0432.Ccr-12-0628
Conti L, Lanzardo S, Arigoni M, Antonazzo R, Radaelli E, Cantarella D, Calogero RA, Cavallo F (2013) The noninflammatory role of high mobility group box 1/Toll-like receptor 2 axis in the self-renewal of mammary cancer stem cells. FASEB J Off Publ Fed Am Soc Exp Biol 27:4731–4744. https://doi.org/10.1096/fj.13-230201
Wang X, Osada T, Wang Y et al (2010) CSPG4 protein as a new target for the antibody-based immunotherapy of triple-negative breast cancer. J Natl Cancer Inst 102:1496–1512. https://doi.org/10.1093/jnci/djq343
Wang Y, Geldres C, Ferrone S, Dotti G (2015) Chondroitin sulfate proteoglycan 4 as a target for chimeric antigen receptor-based T-cell immunotherapy of solid tumors. Expert Opin Ther Targets 19:1339–1350. https://doi.org/10.1517/14728222.2015.1068759
Mittelman A, Chen GZ, Wong GY, Liu C, Hirai S, Ferrone S (1995) Human high molecular weight-melanoma associated antigen mimicry by mouse anti-idiotypic monoclonal antibody MK2-23: modulation of the immunogenicity in patients with malignant melanoma. Clinical Cancer Res Off J Am Assoc Cancer Res 1:705–713
Wang X, Ko EC, Peng L, Gillies SD, Ferrone S (2005) Human high molecular weight melanoma-associated antigen mimicry by mouse anti-idiotypic monoclonal antibody MK2-23: enhancement of immunogenicity of anti-idiotypic monoclonal antibody MK2-23 by fusion with interleukin 2. Cancer Res 65:6976–6983. https://doi.org/10.1158/0008-5472.CAN-04-2328
Quaglino E, Riccardo F, Macagno M, Bandini S, Cojoca R, Ercole E, Amici A, Cavallo F (2011) Chimeric DNA vaccines against ErbB2 + carcinomas: from mice to humans. Cancers 3:3225–3241. https://doi.org/10.3390/cancers3033225
Aurisicchio L, Mancini R, Ciliberto G (2013) Cancer vaccination by electro-gene-transfer. Expert Rev Vaccines 12:1127–1137. https://doi.org/10.1586/14760584.2013.836903
Impellizeri JA, Gavazza A, Greissworth E, Crispo A, Montella M, Ciliberto G, Lubas G, Aurisicchio L (2018) Tel-eVax: a genetic vaccine targeting telomerase for treatment of canine lymphoma. J Transl Med 16:349. https://doi.org/10.1186/s12967-018-1738-6
Gavazza A, Lubas G, Fridman A et al (2013) Safety and efficacy of a genetic vaccine targeting telomerase plus chemotherapy for the therapy of canine B-cell lymphoma. Human Gene Ther 24:728–738. https://doi.org/10.1089/hum.2013.112
Peruzzi D, Gavazza A, Mesiti G et al (2010) A vaccine targeting telomerase enhances survival of dogs affected by B-cell lymphoma. Mol Ther J Am Soc Gene Ther 18:1559–1567. https://doi.org/10.1038/mt.2010.104
Marconato L, Stefanello D, Sabattini S et al (2015) Enhanced therapeutic effect of APAVAC immunotherapy in combination with dose-intense chemotherapy in dogs with advanced indolent B-cell lymphoma. Vaccine 33:5080–5086. https://doi.org/10.1016/j.vaccine.2015.08.017
Milevoj N, Tratar UL, Nemec A, Brozic A, Znidar K, Sersa G, Cemazar M, Tozon N (2019) A combination of electrochemotherapy, gene electrotransfer of plasmid encoding canine IL-12 and cytoreductive surgery in the treatment of canine oral malignant melanoma. Res Vet Sci 122:40–49. https://doi.org/10.1016/j.rvsc.2018.11.001
Kurupati RK, Zhou X, Xiang Z, Keller LH, Ertl HCJ (2018) Safety and immunogenicity of a potential checkpoint blockade vaccine for canine melanoma. Cancer Immunol Immunother CII 67:1533–1544. https://doi.org/10.1007/s00262-018-2201-5
Finocchiaro LM, Fondello C, Gil-Cardeza ML, Rossi UA, Villaverde MS, Riveros MD, Glikin GC (2015) Cytokine-enhanced vaccine and interferon-beta plus suicide gene therapy as surgery adjuvant treatments for spontaneous canine melanoma. Human Gene Ther 26:367–376. https://doi.org/10.1089/hum.2014.130
Riccardo F, Bolli E, Macagno M, Arigoni M, Cavallo F, Quaglino E (2017) Chimeric DNA vaccines: an effective way to overcome immune tolerance. Curr Top Microbiol Immunol 405:99–122. https://doi.org/10.1007/82_2014_426
Acknowledgements
Monoclonal antibodies directed towards different epitopes of the CSPG4 antigen (225.2, TP32, TP49 and VF20-VT87.41) used to perform flow cytometry analysis were kindly provided by Prof. Soldano Ferrone (Department of Surgery, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA). We thank Dr. Dale Lawson for his revision and editing of the manuscript.
Funding
This work was supported by Grants from Fondazione Ricerca Molinette Onlus, the University of Turin (ex 60% 2018, intramural funds) and the Italian Ministry of Health (Progetti ordinari di Ricerca Finalizzata, RF-2013-02359216). FR was supported by a fellowship from Fondazione Italiana per la Ricerca sul Cancro (FIRC).
Author information
Authors and Affiliations
Contributions
LT, GB, SI, DG and FR produced the results discussed in this review. FR, LT and GB performed mouse experiments and flow cytometry analysis, supervised by FC. SI and DG, under the supervision of PB, collaborated to produce the results in canine patients. FC, FR and LT provided major contributions in writing and discussing the manuscript. FC and EQ critically revised the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that no potential conflicts of interest exist.
Ethical approval and ethical standards
All the in vivo experiments were approved by the Italian Ministry of Health, authorization numbers 0006939-P-18/03/2015 (164/2015-PR) and 0004230-20/02/2018-DGSAF-MDS-P.
Animal source
Mice used for the vaccination experiments reported in this paper were purchased from Charles River Laboratories or bred at the Molecular Biotechnology Center, University of Turin, where all mice were maintained and treated in accordance with University Ethical Committee and European Union guidelines under Directive 2010/63. The canine patients that were enrolled in veterinary trials were client-owned dogs, whose institutes of reference were the Veterinary Teaching Hospital of the University of Turin and the Veterinary clinics of South Rome, Italy. Dogs were treated according to the Good Clinical Practice guidelines for animal clinical studies, and rules imposed by the Ethical Committee of the University of Turin (Italy).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tarone, L., Barutello, G., Iussich, S. et al. Naturally occurring cancers in pet dogs as pre-clinical models for cancer immunotherapy. Cancer Immunol Immunother 68, 1839–1853 (2019). https://doi.org/10.1007/s00262-019-02360-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-019-02360-6