Abstract
Background
Immunotherapy has raised the issue of appropriate treatment response evaluation, due to the unique mechanism of action of the immunotherapeutic agents. Aim of this analysis is to evaluate the potential role of quantitative analysis of 2-deoxy-2-(18F)fluoro-d-glucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) data in monitoring of patients with metastatic melanoma undergoing ipilimumab therapy.
Methods
25 patients with unresectable metastatic melanoma underwent dynamic PET/CT (dPET/CT) of the thorax and upper abdomen as well as static, whole body PET/CT with 18F-FDG before the start of ipilimumab treatment (baseline PET/CT), after two cycles of treatment (interim PET/CT) and at the end of treatment after four cycles (late PET/CT). The evaluation of dPET/CT studies was based on semi-quantitative (standardized uptake value, SUV) calculation as well as quantitative analysis, based on two-tissue compartment modeling and a fractal approach. Patients’ best clinical response, assessed at a mean of 59 weeks, was used as reference.
Results
According to their best clinical response, patients were dichotomized in those demonstrating clinical benefit (CB, n = 16 patients) and those demonstrating no clinical benefit (no-CB, n = 9 patients). No statistically significant differences were observed between CB and no-CB regarding either semi-quantitative or quantitative parameters in all scans. On contrary, the application of the recently introduced PET response evaluation criteria for immunotherapy (PERCIMT) led to a correct classification rate of 84% (21/25 patients).
Conclusion
Quantitative analysis of 18F-FDG PET data does not provide additional information in treatment response evaluation of metastatic melanoma patients receiving ipilimumab. PERCIMT criteria correlated better with clinical response.



Similar content being viewed by others
Abbreviations
- 18F-FDG:
-
2-Deoxy-2-(18F)fluoro-d-glucose
- CB:
-
Clinical benefit
- CMR:
-
Complete metabolic response
- CR:
-
Complete response
- CT:
-
Computed tomography
- dPET/CT:
-
Dynamic positron emission tomography/computed tomography
- FD:
-
Fractal dimension
- iCPD:
-
Confirmed progressive disease
- irRC:
-
Immune-related response criteria
- iUPD:
-
Unconfirmed progressive disease
- MB:
-
Metabolic benefit
- MIP:
-
Maximum intensity projection
- No-CB:
-
No clinical benefit
- No-MB:
-
No metabolic benefit
- PD:
-
Progressive disease
- PECRIT PET/CT:
-
Criteria for early prediction of response to immune checkpoint inhibitor therapy
- PERCIMT:
-
PET response evaluation criteria for immunotherapy
- PERCIST:
-
PET response criteria in solid tumors
- PET:
-
Positron emission tomography
- PET/CT:
-
Positron emission tomography/computed tomography
- PMD:
-
Progressive metabolic disease
- PMR:
-
Partial metabolic response
- PR:
-
Partial response
- SD:
-
Stable disease
- SMD:
-
Stable metabolic disease
- SUV:
-
Standardized uptake value
- TAC:
-
Time activity curve
- VOI:
-
Volume of interest
References
Brunet JF, Denizot F, Luciani MF et al (1987) A new member of the immunoglobulin superfamily—CTLA-4. Nature 328:267–270. https://doi.org/10.1038/328267a0
Schneider H, Downey J, Smith A et al (2006) Reversal of the TCR Stop Signal by CTLA-4. Science 313:1972–1975. https://doi.org/10.1126/science.1131078
Hodi FS, O’Day SJ, McDermott DF et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723. https://doi.org/10.1056/NEJMoa1003466
Robert C, Thomas L, Bondarenko I et al (2011) Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 364:2517–2526. https://doi.org/10.1056/NEJMoa1104621
Postow MA, Chesney J, Pavlick AC et al (2015) Nivolumab and Ipilimumab versus Ipilimumab in Untreated Melanoma. N Engl J Med 372:2006–2017. https://doi.org/10.1056/NEJMoa1414428
Wolchok JD, Chiarion-Sileni V, Gonzalez R et al (2017) Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 377:1345–1356. https://doi.org/10.1056/NEJMoa1709684
Couzin-Frankel J (2013) Breakthrough of the year 2013. Cancer immunotherapy. Science 342:1432–1433. https://doi.org/10.1126/science.342.6165.1432
Gilardi L, Grana CM, Paganelli G (2014) Evaluation of response to immunotherapy: new challenges and opportunities for PET imaging. Eur J Nucl Med Mol Imaging 41:2090–2092. https://doi.org/10.1007/s00259-014-2848-x
Phelps ME, Huang SC, Hoffman EJ et al (1979) Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-d-glucose: validation of method. Ann Neurol 6:371–388. https://doi.org/10.1002/ana.410060502
Dimitrakopoulou-Strauss A, Strauss LG, Burger C et al (2003) On the fractal nature of positron emission tomography (PET) studies. World J Nucl Med 4:306–313
Sachpekidis C, Larribere L, Pan L et al (2014) Predictive value of early 18F-FDG PET/CT studies for treatment response evaluation to ipilimumab in metastatic melanoma: preliminary results of an ongoing study. Eur J Nucl Med Mol Imaging 42:386–396. https://doi.org/10.1007/s00259-014-2944-y
Cho SY, Lipson EJ, Im H-J et al (2017) Prediction of response to immune checkpoint inhibitor therapy using early-time-point 18F-FDG PET/CT imaging in patients with advanced melanoma. J Nucl Med 58:1421–1428. https://doi.org/10.2967/jnumed.116.188839
Seith F, Forschner A, Schmidt H et al (2018) 18F-FDG-PET detects complete response to PD1-therapy in melanoma patients two weeks after therapy start. Eur J Nucl Med Mol Imaging 45:95–101. https://doi.org/10.1007/s00259-017-3813-2
Anwar H, Sachpekidis C, Winkler J et al (2018) Absolute number of new lesions on 18F-FDG PET/CT is more predictive of clinical response than SUV changes in metastatic melanoma patients receiving ipilimumab. Eur J Nucl Med Mol Imaging 45:376–383. https://doi.org/10.1007/s00259-017-3870-6
Strauss LG, Conti PS (1991) The applications of PET in clinical oncology. J Nucl Med 32:623–648
PMOD Technologies. http://www.pmod.com/files/download/v31/doc/pbas/4729.htm. Accessed 20 October 2017
Sokoloff L, Smith CB (1983) Basic principles underlying radioisotopic methods for assay of biochemical processes in vivo. In: Greitz T, Ingvar DH, Widén L (eds) The metabolism of the human brain studied with positron emission tomography. Raven Press, New York, pp 123–148
Ohtake T, Kosaka N, Watanabe T et al (1991) Noninvasive method to obtain input function for measuring tissue glucose utilization of thoracic and abdominal organs. J Nucl Med 32:1432–1438
Miyazawa H, Osmont A, Petit-Taboué MC et al (1993) Determination of 18F-fluoro-2-deoxy-d-glucose rate constants in the anesthetized baboon brain with dynamic positron tomography. J Neurosci Methods 50:263–272
Burger C, Buck A (1997) Requirements and implementation of a flexible kinetic modeling tool. J Nucl Med 38:1818–1823
Mikolajczyk K, Szabatin M, Rudnicki P (1998) et al A JAVA environment for medical image data analysis: initial application for brain PET quantitation. Med Inform (Lond) 23:207–214
Sachpekidis C, Mai EK, Goldschmidt H et al (2015) (18)F-FDG dynamic PET/CT in patients with multiple myeloma: patterns of tracer uptake and correlation with bone marrow plasma cell infiltration rate. Clin Nucl Med 40:e300–e307. https://doi.org/10.1097/RLU.0000000000000773
Wolchok JD, Hoos A, O’Day S et al (2009) Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 15:7412–7420. https://doi.org/10.1158/1078-0432.CCR-09-1624
Hodi FS, Butler M, Oble DA et al (2008) Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci 105:3005–3010. https://doi.org/10.1073/pnas.0712237105
Dougan M, Dranoff G (2009) Immune therapy for cancer. Annu Rev Immunol 27:83–117. https://doi.org/10.1146/annurev.immunol.021908.132544
Hersh EM, O’Day SJ, Powderly J et al (2011) A phase II multicenter study of ipilimumab with or without dacarbazine in chemotherapy-naïve patients with advanced melanoma. Invest New Drugs 29:489–498. https://doi.org/10.1007/s10637-009-9376-8
Pennock GK, Waterfield W, Wolchok JD (2012) Patient responses to ipilimumab, a novel immunopotentiator for metastatic melanoma: how different are these from conventional treatment responses? Am J Clin Oncol 35:606–611. https://doi.org/10.1097/COC.0b013e318209cda9
Tirumani SH, Ramaiya NH, Keraliya A et al (2015) Radiographic profiling of immune-related adverse events in advanced melanoma patients treated with ipilimumab. Cancer Immunol Res 3:1185–1192. https://doi.org/10.1158/2326-6066.CIR-15-0102
Amin A, Lawson DH, Salama AKS et al (2016) Phase II study of vemurafenib followed by ipilimumab in patients with previously untreated BRAF-mutated metastatic melanoma. J Immunother Cancer 4:44. https://doi.org/10.1186/s40425-016-0148-7
Seymour L, Bogaerts J, Perrone A et al (2017) iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 18:e143–e152. https://doi.org/10.1016/S1470-2045(17)30074-8
Holder WD, White RL, Zuger JH et al (1998) Effectiveness of positron emission tomography for the detection of melanoma metastases. Ann Surg 227:764–769
Dietlein M, Krug B, Groth W et al (1999) Positron emission tomography using 18F-fluorodeoxyglucose in advanced stages of malignant melanoma: a comparison of ultrasonographic and radiological methods of diagnosis. Nucl Med Commun 20:255–261
Eigtved A, Andersson AP, Dahlstrøm K et al (2000) Use of fluorine-18 fluorodeoxyglucose positron emission tomography in the detection of silent metastases from malignant melanoma. Eur J Nucl Med 27:70–75
Mijnhout GS, Hoekstra OS, van Tulder MW et al (2001) Systematic review of the diagnostic accuracy of (18)F-fluorodeoxyglucose positron emission tomography in melanoma patients. Cancer 91:1530–1542
Swetter SM, Carroll LA, Johnson DL, Segall GM (2002) Positron emission tomography is superior to computed tomography for metastatic detection in melanoma patients. Ann Surg Oncol 9:646–653
Fuster D, Chiang S, Johnson G et al (2004) Is 18F-FDG PET more accurate than standard diagnostic procedures in the detection of suspected recurrent melanoma? J Nucl Med 45:1323–1327
Strobel K, Skalsky J, Hany TF et al (2007) Small bowel invagination caused by intestinal melanoma metastasis: unsuspected diagnosis by FDG-PET/CT imaging. Clin Nucl Med 32:213–214. https://doi.org/10.1097/01.rlu.0000255212.17086.e9
Xing Y, Bronstein Y, Ross MI et al (2011) Contemporary diagnostic imaging modalities for the staging and surveillance of melanoma patients: a meta-analysis. J Natl Cancer Inst 103:129–142. https://doi.org/10.1093/jnci/djq455
Danielsen M, Højgaard L, Kjær A, Fischer BM (2013) Positron emission tomography in the follow-up of cutaneous malignant melanoma patients: a systematic review. Am J Nucl Med Mol Imaging 4:17–28
Peck M, Pollack HA, Friesen A et al (2015) Applications of PET imaging with the proliferation marker [18F]-FLT. Q J Nucl Med Mol Imaging 59:95–104
Ribas A, Benz MR, Allen-Auerbach MS et al (2010) Imaging of CTLA4 blockade-induced cell replication with (18)F-FLT PET in patients with advanced melanoma treated with tremelimumab. J Nucl Med 51:340–346. https://doi.org/10.2967/jnumed.109.070946
Maute RL, Gordon SR, Mayer AT et al (2015) Engineering high-affinity PD-1 variants for optimized immunotherapy and immuno-PET imaging. Proc Natl Acad Sci 112:E6506–E6514. https://doi.org/10.1073/pnas.1519623112
Tavare R, McCracken MN, Zettlitz KA et al (2014) Engineered antibody fragments for immuno-PET imaging of endogenous CD8+ T cells in vivo. Proc Natl Acad Sci 111:1108–1113. https://doi.org/10.1073/pnas.1316922111
Tavaré R, Escuin-Ordinas H, Mok S et al (2016) An effective immuno-PET imaging method to monitor CD8-dependent responses to immunotherapy. Cancer Res 76:73–82. https://doi.org/10.1158/0008-5472.CAN-15-1707
Larimer BM, Wehrenberg-Klee E, Dubois F et al (2017) Granzyme B PET imaging as a predictive biomarker of immunotherapy response. Cancer Res 77:2318–2327. https://doi.org/10.1158/0008-5472.CAN-16-3346
Mayer AT, Natarajan A, Gordon SR et al (2017) Practical immuno-PET radiotracer design considerations for human immune checkpoint imaging. J Nucl Med 58:538–546. https://doi.org/10.2967/jnumed.116.177659
Guldbrandsen KF, Hendel HW, Langer SW, Fischer BM (2017) Nuclear molecular imaging strategies in immune checkpoint inhibitor therapy. Diagnostics (Basel). https://doi.org/10.3390/diagnostics7020023
Dimitrakopoulou-Strauss A, Pan L, Strauss LG (2012) Quantitative approaches of dynamic FDG-PET and PET/CT studies (dPET/CT) for the evaluation of oncological patients. Cancer Imaging 12:283–289. https://doi.org/10.1102/1470-7330.2012.0033
Funding
This study was supported in part by the German Cancer Aid under the project with the title “Therapy monitoring of ipilimumab” based on the quantification of F-18-FDG kinetics with 4D PET/CT (dPET-CT) in patients with melanoma (stage 4). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
Author information
Authors and Affiliations
Contributions
CS performed the PET/CT studies, carried out the PET/CT data analysis and drafted the manuscript. HA performed the PET/CT studies. JKW performed the ipilimumab therapies. AKS was responsible for the statistical analysis of the study. LL contributed to draft the manuscript. UH participated in the design of the study. JCH was responsible for the selection of the patients who received the ipilimumab therapy. ADS was responsible for the PET-CT study design and the data evaluation, coordinated the project and contributed to the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Christos Sachpekidis reports travel grants from Janssen-Cilag outside the submitted work. Julia Winkler received speakers honoraria from Merck Sharp & Dohme (MSD), and travel support from AMGEN, Bristol-Myers Squibb (BMS), MSD, Philochem and Roche. Jessica C. Hassel received honoraria for talks and travel expenses from BMS, MSD, Roche, Novartis, Pfizer and is a member of an advisory board for MSD and Amgen. The other authors declare that they have no conflict of interest.
Ethical approval
Patients gave written informed consent to participate in the study and to have their medical records released. The study was approved by the Ethical Committee of the University of Heidelberg and the Federal Agency for Radiation Protection (Bundesamt für Strahlenschutz).
Informed consent
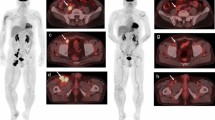
Informed consent was obtained from all individual participants included in the study. The patients presented on Figs. 2 and 3 agreed on the publication of these figures. This study does not contain any studies with animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sachpekidis, C., Anwar, H., Winkler, J.K. et al. Longitudinal studies of the 18F-FDG kinetics after ipilimumab treatment in metastatic melanoma patients based on dynamic FDG PET/CT. Cancer Immunol Immunother 67, 1261–1270 (2018). https://doi.org/10.1007/s00262-018-2183-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-018-2183-3