Abstract
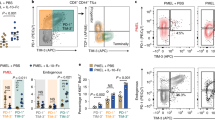
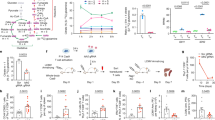
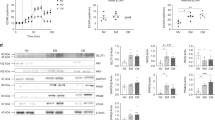
Adoptive cell therapy (ACT) employing ex vivo-generated tumor antigen-specific CD8+ T cells shows tumor efficacy when the transferred cells possess both effector and memory functions. New strategies based on understanding of mechanisms that balance CD8+ T cell differentiation toward effector and memory responses are highly desirable. Emerging information confirms a central role for antigen-induced metabolic reprogramming in CD8+ T cell differentiation and clonal expansion. The mitochondrial protein uncoupling protein 2 (UCP2) is induced by antigen stimulation of CD8+ T cells; however, its role in metabolic reprogramming underlying differentiation and clonal expansion has not been reported. Employing genetic (siRNA) and pharmacologic (Genipin) approaches, we note that antigen-induced UCP2 expression reduces glycolysis, fatty acid synthesis and production of reactive oxygen species to balance differentiation with survival of effector CD8+ T cells. Inhibition of UCP2 promotes CD8+ T cell terminal differentiation into short-lived effector cells (CD62LloKLRG1HiIFNγHi) that undergo clonal contraction. These findings are the first to reveal a role for antigen-induced UCP2 expression in balancing CD8+ T cell differentiation and survival. Targeting UCP2 to regulate metabolic reprogramming of CD8+ T cells is an attractive new approach to augment efficacy of tumor therapy by ACT.



Similar content being viewed by others
Abbreviations
- ACT:
-
Adoptive cell therapy
- CM-H2DCFDA:
-
5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester
- cROS:
-
Cytoplasmic reactive oxygen species
- CTLA-4:
-
Cytotoxic T-lymphocyte-associated protein 4
- ECAR:
-
Extracellular acidification rate
- ETC:
-
Electron transport chain
- FAO:
-
Fatty acid oxidation
- FAS:
-
Fatty acid synthesis
- mROS:
-
Mitochondrial reactive oxygen species
- NAD+/NADH:
-
Nicotinamide adenine dinucleotide/dehydrogenase
- OCR:
-
Oxygen consumption rate
- OXPHOS:
-
Oxidative phosphorylation
- PCR:
-
Polymerase chain reaction
- PD-1/PD-L1:
-
Programmed cell death protein 1/ligand
- PDH:
-
Pyruvate dehydrogenase
- ROS:
-
Reactive oxygen species
- SRC:
-
Spare respiratory capacity
- TCA:
-
Tricarboxylic acid cycle
- TCR:
-
T cell receptor
- UCP2:
-
Uncoupling protein 2
References
Buck MD, O’Sullivan D, Pearce EL (2015) T cell metabolism drives immunity. J Exp Med 212(9):1345–1360. doi:10.1084/jem.20151159
Lunt SY, Vander Heiden MG (2011) Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol 27:441–464. doi:10.1146/annurev-cellbio-092910-154237
MacIver NJ, Michalek RD, Rathmell JC (2013) Metabolic regulation of T lymphocytes. Annu Rev Immunol 31:259–283. doi:10.1146/annurev-immunol-032712-095956
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324(5930):1029–1033. doi:10.1126/science.1160809
Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, Wang CR, Schumacker PT, Licht JD, Perlman H, Bryce PJ, Chandel NS (2013) Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 38(2):225–236. doi:10.1016/j.immuni.2012.10.020
Devadas S, Zaritskaya L, Rhee SG, Oberley L, Williams MS (2002) Discrete generation of superoxide and hydrogen peroxide by T cell receptor stimulation: selective regulation of mitogen-activated protein kinase activation and fas ligand expression. J Exp Med 195(1):59–70
Murphy MP, Siegel RM (2013) Mitochondrial ROS fire up T cell activation. Immunity 38(2):201–202. doi:10.1016/j.immuni.2013.02.005
Wang R, Green DR (2012) Metabolic checkpoints in activated T cells. Nat Immunol 13(10):907–915. doi:10.1038/ni.2386
van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, Pearce EJ, Pearce EL (2012) Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36(1):68–78. doi:10.1016/j.immuni.2011.12.007
Criscuolo F, Mozo J, Hurtaud C, Nubel T, Bouillaud F (2006) UCP2, UCP3, avUCP, what do they do when proton transport is not stimulated? Possible relevance to pyruvate and glutamine metabolism. Biochim Biophys Acta 1757(9–10):1284–1291. doi:10.1016/j.bbabio.2006.06.002
Pecqueur C, Alves-Guerra C, Ricquier D, Bouillaud F (2009) UCP2, a metabolic sensor coupling glucose oxidation to mitochondrial metabolism? IUBMB Life 61(7):762–767. doi:10.1002/iub.188
Pecqueur C, Bui T, Gelly C, Hauchard J, Barbot C, Bouillaud F, Ricquier D, Miroux B, Thompson CB (2008) Uncoupling protein-2 controls proliferation by promoting fatty acid oxidation and limiting glycolysis-derived pyruvate utilization. FASEB J 22(1):9–18. doi:10.1096/fj.07-8945com
Arsenijevic D, Onuma H, Pecqueur C, Raimbault S, Manning BS, Miroux B, Couplan E, Alves-Guerra MC, Goubern M, Surwit R, Bouillaud F, Richard D, Collins S, Ricquier D (2000) Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet 26(4):435–439. doi:10.1038/82565
Bai Y, Onuma H, Bai X, Medvedev AV, Misukonis M, Weinberg JB, Cao W, Robidoux J, Floering LM, Daniel KW, Collins S (2005) Persistent nuclear factor-kappa B activation in Ucp2-/- mice leads to enhanced nitric oxide and inflammatory cytokine production. J Biol Chem 280(19):19062–19069. doi:10.1074/jbc.M500566200
Kizaki T, Suzuki K, Hitomi Y, Taniguchi N, Saitoh D, Watanabe K, Onoe K, Day NK, Good RA, Ohno H (2002) Uncoupling protein 2 plays an important role in nitric oxide production of lipopolysaccharide-stimulated macrophages. Proc Natl Acad Sci USA 99(14):9392–9397. doi:10.1073/pnas.142206299
Rupprecht A, Brauer AU, Smorodchenko A, Goyn J, Hilse KE, Shabalina IG, Infante-Duarte C, Pohl EE (2012) Quantification of uncoupling protein 2 reveals its main expression in immune cells and selective up-regulation during T-cell proliferation. PLoS One 7(8):e41406. doi:10.1371/journal.pone.0041406
Kaminski M, Kiessling M, Suss D, Krammer PH, Gulow K (2007) Novel role for mitochondria: protein kinase Ctheta-dependent oxidative signaling organelles in activation-induced T-cell death. Mol Cell Biol 27(10):3625–3639. doi:10.1128/mcb.02295-06
Bouillaud F (2009) UCP2, not a physiologically relevant uncoupler but a glucose sparing switch impacting ROS production and glucose sensing. Biochim Biophys Acta 1787(5):377–383. doi:10.1016/j.bbabio.2009.01.003
Emre Y, Nubel T (2010) Uncoupling protein UCP2: when mitochondrial activity meets immunity. FEBS Lett 584(8):1437–1442. doi:10.1016/j.febslet.2010.03.014
Locasale JW, Cantley LC (2011) Metabolic flux and the regulation of mammalian cell growth. Cell Metab 14(4):443–451. doi:10.1016/j.cmet.2011.07.014
Joseph JW, Jensen MV, Ilkayeva O, Palmieri F, Alarcon C, Rhodes CJ, Newgard CB (2006) The mitochondrial citrate/isocitrate carrier plays a regulatory role in glucose-stimulated insulin secretion. J Biol Chem 281(47):35624–35632. doi:10.1074/jbc.M602606200
Zhang J, Khvorostov I, Hong JS, Oktay Y, Vergnes L, Nuebel E, Wahjudi PN, Setoguchi K, Wang G, Do A, Jung HJ, McCaffery JM, Kurland IJ, Reue K, Lee WN, Koehler CM, Teitell MA (2011) UCP2 regulates energy metabolism and differentiation potential of human pluripotent stem cells. EMBO J 30(24):4860–4873. doi:10.1038/emboj.2011.401
Vozza A, Parisi G, De Leonardis F, Lasorsa FM, Castegna A, Amorese D, Marmo R, Calcagnile VM, Palmieri L, Ricquier D, Paradies E, Scarcia P, Palmieri F, Bouillaud F, Fiermonte G (2014) UCP2 transports C4 metabolites out of mitochondria, regulating glucose and glutamine oxidation. Proc Natl Acad Sci USA 111(3):960–965. doi:10.1073/pnas.1317400111
Weinberg SE, Sena LA, Chandel NS (2015) Mitochondria in the regulation of innate and adaptive immunity. Immunity 42(3):406–417. doi:10.1016/j.immuni.2015.02.002
Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, Paulos CM, Muranski P, Restifo NP (2009) Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med 15(7):808–813. doi:10.1038/nm.1982
Wherry EJ, Teichgrӓber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R (2003) Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol 4(3):225–234. doi:10.1038/ni889
Sukumar M, Liu J, Ji Y, Subramanian M, Crompton JG, Yu Z, Roychoudhuri R, Palmer DC, Muranski P, Karoly ED, Mohney RP, Klebanoff CA, Lal A, Finkel T, Restifo NP, Gattinoni L (2013) Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J Clin Invest 123(10):4479–4488. doi:10.1172/jci69589
Topalian SL, Drake CG, Pardoll DM (2015) Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27(4):450–461. doi:10.1016/j.ccell.2015.03.001
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12(4):252–264. doi:10.1038/nrc3239
Patsoukis N, Bardhan K, Chatterjee P, Sari D, Liu B, Bell LN, Karoly ED, Freeman GJ, Petkova V, Seth P, Li L, Boussiotis VA (2015) PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun 6:6692. doi:10.1038/ncomms7692
Acknowledgments
We thank all current and past members of the Shrikant laboratory for critical review, comments and discussions. This work was supported by the NIH National Cancer Institute (NCI) (P50CA158318-3, Protul A. Shrikant).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial conflicts of interest.
Additional information
This paper is a Focussed Research Review based on a presentation given at the Fourth International Conference on Cancer Immunotherapy and Immunomonitoring (CITIM 2015), held in Ljubljana, Slovenia, 27th–30th April 2015. It is part of a series of Focussed Research Reviews and meeting report in Cancer Immunology, Immunotherapy.
Rights and permissions
About this article
Cite this article
Chaudhuri, L., Srivastava, R.K., Kos, F. et al. Uncoupling protein 2 regulates metabolic reprogramming and fate of antigen-stimulated CD8+ T cells. Cancer Immunol Immunother 65, 869–874 (2016). https://doi.org/10.1007/s00262-016-1851-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-016-1851-4