Abstract
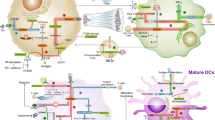
Targeting the tumor microenvironment focusing on immune cells has recently become a standard of care for some tumors. Indeed, antibodies blocking immune checkpoints (e.g., anti-CTLA-4 and anti-PD1 mAbs) have been approved by regulatory agencies for the treatment of some solid tumors based upon successes in many clinical trials. Although tumor metabolism has always attracted the attention of tumor biologists, only recently have oncologists renewed their interest in this field of tumor biology research. This has highlighted the possibility to pharmacologically target rate-limiting enzymes along key metabolic pathways of tumor cells, such as lipogenesis and aerobic glycolysis. Altered tumor metabolism has also been shown to influence the functionality of the tumor microenvironment as a whole, particularly the immune cell component of thereof. Cholesterol, oxysterols and Liver X receptors (LXRs) have been investigated in different tumor models. Recent in vitro and in vivo results point to their involvement in tumor and immune cell biology, thus making the LXR/oxysterol axis a possible target for novel antitumor strategies. Indeed, the possibility to target both tumor cell metabolism (i.e., cholesterol metabolism) and tumor-infiltrating immune cell dysfunctions induced by oxysterols might result in a synergistic antitumor effect generating long-lasting memory responses. This review will focus on the role of cholesterol metabolism with particular emphasis on the role of the LXR/oxysterol axis in the tumor microenvironment, discussing mechanisms of action, pros and cons, and strategies to develop antitumor therapies based on the modulation of this axis.
Similar content being viewed by others
Abbreviations
- ABC:
-
ATP-binding cassette
- AIM/SPα:
-
Apoptosis inhibitor of macrophages
- BRAF:
-
BRAF proto-oncogene, serine/threonine kinase
- CCR:
-
Chemokine receptor
- CTLA-4:
-
Cytotoxic T lymphocyte-associated protein 4
- CXCR:
-
CXC chemokine receptor
- DC:
-
Dendritic cell
- ER:
-
Endoplasmic reticulum
- HDL:
-
High-density lipoprotein
- HMGCR:
-
Hydroxyl-methyl glutaryl-coenzyme A reductase
- INSIG:
-
Insulin-induced gene
- LDL:
-
Low-density lipoprotein
- LDLR:
-
Low-density lipoprotein receptor
- LXR:
-
Liver X receptor
- mTOR:
-
Mammalian target of rapamycin
- PD1:
-
Programmed cell death protein 1
- RCT:
-
Reverse cholesterol transport
- SCAP:
-
SREBP-cleavage activation protein
- SREBP:
-
Sterol response element binding protein
- SULT2B1b:
-
Sulfotransferase 2B1b
- Th:
-
T helper cell
References
Topalian SL, Drake CG, Pardoll DM (2015) Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27:450–461. doi:10.1016/j.ccell.2015.03.00
Postow MA, Callahan MK, Wolchok JD (2015) Immune checkpoint blockade in cancer therapy. J Clin Oncol 33:1974–1982. doi:10.1200/JCO.2014.59.4358
Sun Y (2015) Translational horizons in the tumor microenvironment: harnessing breakthroughs and targeting cures. Med Res Rev 35:408–436. doi:10.1002/med.21338
Sullivan RJ, Flaherty K (2012) MAP kinase signaling and inhibition in melanoma. Oncogene 32:2373–2379. doi:10.1038/onc.2012.345
Sharma P, Wagner K, Wolchok JD, Allison JP (2011) Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer 11:805–812. doi:10.1038/nrc3153
Shin DS, Ribas A (2015) The evolution of checkpoint blockade as a cancer therapy: what’s here, what’s next? Curr Opin Immunol 33:23–35. doi:10.1016/j.coi.2015.01.006
DeBerardinis RJ, Thompson CB (2012) Cellular metabolism and disease: what do metabolic outliers teach us? Cell 148:1132–1144. doi:10.1016/j.cell.2012.02.032
Villalba M, Rathore MG, Lopez-Royuela N, Krzywinska E, Garaude J, Allende-Vega N (2013) From tumor cell metabolism to tumor immune escape. Int J Biochem Cell Biol 45:106–113. doi:10.1016/j.biocel.2012.04.024
Cairns RA, Harris IS, Mak TW (2011) Regulation of cancer cell metabolism. Nat Rev Cancer 11:85–95. doi:10.1038/nrc2981
Ghesquiere B, Wong BW, Kuchnio A, Carmeliet P (2014) Metabolism of stromal and immune cells in health and disease. Nature 511:167–176. doi:10.1038/nature13312
Warburg O (1956) On respiratory impairment in cancer cells. Science 124:269–270
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324:1029–1033. doi:10.1126/science.1160809
Elstrom RL, Bauer DE, Buzzai M et al (2004) Akt stimulates aerobic glycolysis in cancer cells. Cancer Res 64:3892–3899
Zoncu R, Efeyan A, Sabatini DM (2011) mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12:21–35. doi:10.1038/nrm3025
Menendez JA, Lupu R (2007) Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 7:763–777
Rysman E, Brusselmans K, Scheys K et al (2010) De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res 70:8117–8126
Yuan TL, Cantley LC (2008) PI3K pathway alterations in cancer: variations on a theme. Oncogene 27:5497–5510
Repa JJ, Mangelsdorf DJ (2000) The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu Rev Cell Dev Biol 16:459–481
Chang CH, Qiu J, O’Sullivan D et al (2015) Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162:1229–1241
Ho PC, Bihuniak JD, Macintyre AN et al (2015) Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell 162:1217–1228
Colegio OR, Chu NQ, Szabo AL et al (2014) Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513:559–563
Herber DL, Cao W, Nefedova Y et al (2010) Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med 16:880–886
Cubillos-Ruiz JR, Silberman PC, Rutkowski MR et al (2015) ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell 161:1527–1538
Horton JD, Goldstein JL, Brown MS (2002) SREBPs: transcriptional mediators of lipid homeostasis. Cold Spring Harb Symp Quant Biol 67:491–498
Horton JD, Shah NA, Warrington JA, Anderson NN, Park SW, Brown MS, Goldstein JL (2003) Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc Natl Acad Sci USA 100:12027–12032
Goldstein JL, Brown MS (2015) A century of cholesterol and coronaries: from plaques to genes to statins. Cell 161:161–172
Bovenga F, Sabba C, Moschetta A (2015) Uncoupling nuclear receptor LXR and cholesterol metabolism in cancer. Cell Metab 21:517–526
Wang LJ, Song BL (2012) Niemann–Pick C1-Like 1 and cholesterol uptake. Biochim Biophys Acta 1821:964–972. doi:10.1016/j.bbalip.2012.03.004
Phillips MC (2014) Molecular mechanisms of cellular cholesterol efflux. J Biol Chem 289:24020–24029. doi:10.1074/jbc.R114.583658
Repa JJ, Mangelsdorf DJ (2002) The liver X receptor gene team: potential new players in atherosclerosis. Nat Med 8:1243–1248
Spann NJ, Garmire LX, McDonald JG et al (2012) Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell 151:138–152. doi:10.1016/j.cell.2012.06.054
Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ (1996) An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature 383:728–731
Bjorkhem I (2002) Do oxysterols control cholesterol homeostasis? J Clin Invesig. 110:725–730
Murphy RC, Johnson KM (2008) Cholesterol, reactive oxygen species, and the formation of biologically active mediators. J Biol Chem 283:15521–15525. doi:10.1074/jbc.R700049200
Chen W, Chen G, Head DL, Mangelsdorf DJ, Russell DW (2007) Enzymatic reduction of oxysterols impairs LXR signaling in cultured cells and the livers of mice. Cell Metab 5:73–79
Jakobsson T, Treuter E, Gustafsson JA, Steffensen KR (2012) Liver X receptor biology and pharmacology: new pathways, challenges and opportunities. Trends Pharmacol Sci 33:394–404. doi:10.1016/j.tips.2012.03.013
Zelcer N, Hong C, Boyadjian R, Tontonoz P (2009) LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science 325:100–104. doi:10.1126/science.1168974
Radhakrishnan A, Ikeda Y, Kwon HJ, Brown MS, Goldstein JL (2007) Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: oxysterols block transport by binding to Insig. Proc Natl Acad Sci USA 104:6511–6518
Laffitte BA, Chao LC, Li J et al (2003) Activation of liver X receptor improves glucose tolerance through coordinate regulation of glucose metabolism in liver and adipose tissue. Proc Natl Acad Sci USA 100:5419–5424
Beaven SW, Matveyenko A, Wroblewski K et al (2013) Reciprocal regulation of hepatic and adipose lipogenesis by liver X receptors in obesity and insulin resistance. Cell Metab 18:106–117. doi:10.1016/j.cmet.2013.04.021
Russo V (2011) Metabolism, LXR/LXR ligands, and tumor immune escape. J Leukoc Biol 90:673–679. doi:10.1189/jlb.0411198
Fukuchi J, Kokontis JM, Hiipakka RA, Chuu CP, Liao S (2004) Antiproliferative effect of liver X receptor agonists on LNCaP human prostate cancer cells. Cancer Res 64:7686–7689
Vedin LL, Lewandowski SA, Parini P, Gustafsson JA, Steffensen KR (2009) The oxysterol receptor LXR inhibits proliferation of human breast cancer cells. Carcinogenesis 30:575–579. doi:10.1093/carcin/bgp029
Lo Sasso G, Bovenga F, Murzilli S et al (2013) Liver X receptors inhibit proliferation of human colorectal cancer cells and growth of intestinal tumors in mice. Gastroenterology 144:1497–1507. doi:10.1053/j.gastro.2013.02.005
Nelson ER, Wardell SE, Jasper JS et al (2013) 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science 342:1094–1098. doi:10.1126/science.1241908
Flaveny CA, Griffett K, El-Gendy BE et al (2015) Broad anti-tumor activity of a small molecule that selectively targets the warburg effect and lipogenesis. Cancer Cell 28:42–56. doi:10.1016/j.ccell.2015.05.007
Pencheva N, Buss CG, Posada J, Merghoub T, Tavazoie SF (2014) Broad-spectrum therapeutic suppression of metastatic melanoma through nuclear hormone receptor activation. Cell 156:986–1001. doi:10.1016/j.cell.2014.01.038
Pencheva N, Tran H, Buss C, Huh D, Drobnjak M, Busam K, Tavazoie SF (2012) Convergent multi-miRNA targeting of ApoE drives LRP1/LRP8-dependent melanoma metastasis and angiogenesis. Cell 151:1068–1082. doi:10.1016/j.cell.2012.10.028
Villablanca EJ, Raccosta L, Zhou D et al (2010) Tumor-mediated liver X receptor-alpha activation inhibits CC chemokine receptor-7 expression on dendritic cells and dampens antitumor responses. Nat Med 16:98–105. doi:10.1038/nm.2074
Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P (2003) Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med 9:213–219
Bensinger SJ, Tontonoz P (2008) Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature 454:470–477
Joseph SB, Bradley MN, Castrillo A et al (2004) LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell 119:299–309
Valledor AF, Hsu LC, Ogawa S, Sawka-Verhelle D, Karin M, Glass CK (2004) Activation of liver X receptors and retinoid X receptors prevents bacterial-induced macrophage apoptosis. Proc Natl Acad Sci USA 101:17813–17818
Gonzalez NA, Bensinger SJ, Hong C et al (2009) Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity 31:245–258. doi:10.1016/j.immuni.2009.06.018
Bensinger SJ, Bradley MN, Joseph SB et al (2008) LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell 134:97–111. doi:10.1016/j.cell.2008.04.052
Cui G, Qin X, Wu L et al (2011) Liver X receptor (LXR) mediates negative regulation of mouse and human Th17 differentiation. J Clin Investig 121:658–670. doi:10.1172/JCI42974
Hu X, Wang Y, Hao LY et al (2015) Sterol metabolism controls TH17 differentiation by generating endogenous RORgamma agonists. Nat Chem Biol 11:141–147. doi:10.1038/nchembio.1714
Raccosta L, Fontana R, Maggioni D et al (2013) The oxysterol-CXCR2 axis plays a key role in the recruitment of tumor-promoting neutrophils. J Exp Med 210:1711–1728. doi:10.1084/jem.20130440
Ribas A, Hodi FS, Callahan M, Konto C, Wolchok J (2013) Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med 368:1365–1366. doi:10.1056/NEJMc1302338
Acknowledgments
This work was supported by the Italian Association for Cancer Research (AIRC) and by the Italian Ministry of Health (RF2009).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
This paper is a Focussed Research Review based on a presentation given at the Twelfth Meeting of the Network Italiano per la Bioterapia dei Tumori (NIBIT) on Cancer Bio-Immunotherapy, held in Siena, Italy, 9th–11th October 2014. It is part of a series of Focussed Research Reviews and meeting report in Cancer Immunology, Immunotherapy.
Rights and permissions
About this article
Cite this article
Raccosta, L., Fontana, R., Corna, G. et al. Cholesterol metabolites and tumor microenvironment: the road towards clinical translation. Cancer Immunol Immunother 65, 111–117 (2016). https://doi.org/10.1007/s00262-015-1779-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-015-1779-0