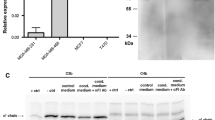
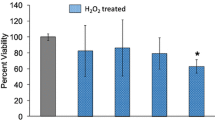
Abstract
Cancer therapies, which deliver a rapidly induced massive tumor tissue injury, such as photodynamic therapy (PDT), provoke a strong host response raised for dealing with the inflicted local trauma. Activated complement system was identified as an important element of host response elicited by tumor PDT. The expression of genes encoding complement proteins C3, C5, and C9 was studied following tumor PDT mediated by photosensitizer Photofrin using mouse Lewis lung carcinoma (LLC) model. Treated tumors and the livers of host mice were collected at different times after PDT and the expression of the investigated genes was analyzed by RT-PCR. The results show a significant up-regulation of C3, C5, and C9 genes in PDT-treated tumors at 24 h after therapy, while no significant increase in the expression of these genes was found in the liver tissues. The expression of C3, C5, and C9 genes also became up-regulated in untreated tumor-associated macrophages (TAMs) co-incubated in vitro with PDT-treated LLC cells. This effect was abolished or drastically reduced in the presence of antibodies blocking heat shock protein 70 (HSP70), Toll-like receptor (TLR) 2 and TLR4, and specific peptide inhibitors of TIRAP adapter protein and transcription factor NF-κB. The presented study reveals that complement genes C3, C5, and C9 become up-regulated in tumors treated by PDT, but not in the host’s liver. Tumor-localized up-regulation of these genes can be largely attributed to monocytes/macrophages invading the treated lesion after PDT. This effect appears to be induced by the recognition of danger signals from PDT-treated tumor cells such as HSP70 by TAMs that involve the TLR2- and TLR4-triggered signal transduction pathways leading to the activation of NF-κB.




Similar content being viewed by others
References
Akira A, Takeda K (2004) Toll-like receptor signaling. Nat Rev Immunol 4:499–511
Alper CA, Johnson AM, Birtch AG, Moore FD (1969) Human C3: evidence for the liver as the primary site of synthesis. Science 163:286–288
Bihl F, Lariviere L, Quereshi ST, Flaherty L, Malo D (2001) LPS-hyporesponsiveness of mnd mice is associated with a mutation in Toll-like receptor 4. Genes Immun 2:56–59
Canti G, De Simone A, Korbelik M (2002) Photodynamic therapy and the immune system in experimental oncology. Photochem Photobiol Sci 1:79–80
Cecic I, Korbelik M (2002) Mediators of peripheral blood neutrophilia induced by photodynamic therapy of solid tumors. Cancer Lett 183:43–51
Cecic I, Serrano K, Gyongyossy-Issa M, Korbelik M (2005) Characteristics of complement activation in mice bearing Lewis lung carcinomas treated by photodynamic therapy. Cancer Lett 225:215–223
Cecic I, Korbelik M (2006) Deposition of complement proteins on cells treated by photodynamic therapy in vitro. J Environ Pathol Toxicol Oncol 25:189–203
Cecic I, Sun J, Korbelik M (2006) Role of complement anaphylatoxin C3a in photodynamic therapy-elicited engagement of host neutrophils and other immune cells. Photochem Photobiol 82:558–562
Cecic I, Stott B, Korbelik M (2006) Acute phase response-associated systemic neutrophil mobilization in mice bearing tumors treated by photodynamic therapy. Int Immunopharmacol 6:1259–1266
Chen WR, Adams RL, Higgins AK, Bartels KE, Nordquist RE (1966) Photothermal effects on murine mammary tumors using indocyanine green and an 808-nm diode laser: an in vivo efficacy study. Cancer Lett 98:169–173
Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q (1998) Photodynamic therapy. J Natl Cancer Inst 90:889–905
Gabay C, Kushner I (1999) Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 340:448–454
Gollnick SO, Liu X, Owczarczak B, Musser DA, Henderson BW (1997) Altered expression of interleukin 6 and interleukin 10 as a result of photodynamic therapy in vivo. Cancer Res 57:3904–3909
Henderson BW, Dougherty TJ (1992) How does photodynamic therapy work? Photochem Photobiol 55:145–157
Huang Z (2005) A review of progress in clinical photodynamic therapy. Technol Cancer Res Treat 4:283–294
Illing RO, Kennedy JE, Wu F, ter Haar GR, Protheroe AS, Friend PJ, Gleeson FV, Cranston DW, Phillips RR, Middleton MR (2005) The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a Western population. Br J Cancer 93:890–895
Ivarsson K, Myllymaki L, Jansner K, Stenram U, Tranberg KG (2005) Resistance to tumour challenge after tumour laser thermotherapy is associated with a cellular immune response. Br J Cancer 93:435–440
Korbelik M, Krosl G (1996) Photofrin accumulation in malignant and host cell populations of various tumours. Br J Cancer 73:506–513
Korbelik M, Sun J, Cecic I (2005) Photodynamic therapy-induced cell surface expression and release of heat shock protein: relevance for tumor response. Cancer Res 65:1018–1026
Korbelik M, Merchant S, Stott B, Cecic I, Payne P, Sun J (2006) Acute phase response induced following tumor treatment by photodynamic therapy: relevance for the therapy outcome. Proc SPIE 6087:60870C1-0870C7
Korbelik M (2006) PDT-associated host response and its role in the therapy outcome. Lasers Surg Med 38:500–508
Krosl G, Korbelik M, Dougherty GJ (1995) Induction of immune cell infiltration into murine SCCVII tumor by Photofrin-based photodynamic therapy. Br J Cancer 71:549–555
Kuflik EG (2004) Cryosurgery for skin cancer: 30-year experience and cure rates. Dermatol Surg 30:297–300
Laufer J, Katz Y, Passwell JH (2001) Extrahepatic synthesis of complement proteins in inflammation. Mol Immunol 38:221–229
Moore FT, Blackwood J, Sanzenbacher L, Pace WG (1968) Cryotherapy for malignant tumors. Immunologic response. Arch Surg 96:527–529
Morgan BP, Gasque P (1997) Extrahepatic complement biosynthesis: where, when and why? Clin Exp Immunol 107:1–7
Morgan BP (2000) The complement system: an overview. In: Morgan BP (ed) Methods in molecular biology, Vol. 150: complement methods and protocols. Humana Press Inc., Totowa, NJ, pp1-3
Naughton MA, Botto M, Carter MJ, Alexander GJM, Goldman JM, Walport MJ (1996) Extrahepatic secreted complement C3 contributes to circulating C3 levels in humans. J Immunol 156:3051–3056
Nauta AJ, Roos A, Daha MR (2004) A regulatory role for complement in innate immunity and autoimmunity. Int Arch Allergy Immunol 134:310–323
Pareek G, Nakada SY (2005) The current role of cryotherapy for renal and prostate tumors. Urol Oncol 23:361–366
Sugiura K, Stock CC (1955) The effect of phosphoramides on the growth of a variety of mouse and rat tumors. Cancer Res 15:38–51
Tranberg KG (2004) Percutaneous ablation of liver tumors. Best Pract Res Clin Gastroenterol 18:125–145
Wu F, Wang ZB, Lu P, Xu ZL, Chen WZ, Zhu H, Jin CB (2004) Activated anti-tumor immunity in cancer patients after high intensity focused ultrasound ablation. Ultrasound Med Biol 30:1217–1222
Wu F, Wang ZB, Zhu H, Chen WZ, Zou JZ, Bai J, Li KQ, Jin CB, Xie FL, Su HB (2005) Extracorporeal high intensity focused ultrasound treatment for patients with breast cancer. Breast Cancer Res Treat 92:51–60
Acknowledgments
The authors wish to thank Dr. Wan Lam for helpful advice in gene expression analysis. This study was supported by the National Cancer Institute of Canada, with funds from the Canadian Cancer Society.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stott, B., Korbelik, M. Activation of complement C3, C5, and C9 genes in tumors treated by photodynamic therapy. Cancer Immunol Immunother 56, 649–658 (2007). https://doi.org/10.1007/s00262-006-0221-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-006-0221-z