Abstract
Purpose
To explore the role of glucose as a stimulation agent in the blood oxygen level-dependent (BOLD) functional magnetic resonance imaging (fMRI) for liver cirrhosis and hepatocellular carcinoma (HCC).
Materials and Methods
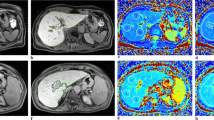
Twenty HCC patients with cirrhosis and 10 healthy volunteers were recruited. BOLD fMRI was performed for all participants prior to and 30 min after oral administration of glucose to measure the T2* values of normal liver parenchyma, HCC liver parenchyma, HCC center, and HCC edge. Variations of the T2*(△T2*) before and after administration were calculated.
Results
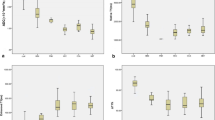
Data from 16 patients and 10 healthy volunteers were reported. Before and after oral administration of glucose, T2* values of the normal liver parenchyma, HCC liver parenchyma, and HCC center were statistically different (p < 0.01), while no statistical difference was found in T2* value of the HCC edge (p = 0.35). △T2* values of the normal liver parenchyma, HCC liver parenchyma, HCC center, and HCC edge were 2.8 ± 1.1 ms, −1.3 ± 1.2 ms, −2.1 ± 1.8 ms, and 0.8 ± 3.2 ms before and after administration, respectively. △T2* value was statistically different in the liver parenchyma between healthy volunteers and HCC patients and between HCC center and HCC edge (both p < 0.01).
Conclusion
Use of glucose as the stimulation agent in BOLD fMRI may facilitate the assessment of liver function for patients with liver cirrhosis. The potential of △T2* to correlate with severity of liver cirrhosis, as well as to evaluate hepatic artery perfusion and bioactivity of HCC center should be further investigated.


Similar content being viewed by others
Reference
He J, Gu D, Wu X, et al. (2005) Major causes of death among men and women in China. N Engl J Med 353:1124–1134
Rastegar RF, Hou D, Harris A, et al. (2012) Is a liver biopsy necessary? Investigation of a suspected hepatocellular carcinoma: a pictorial essay of hepatocellular carcinoma and the revised American Association for the Study of Liver Disease Criteria. Can Assoc Radiol J 63:329–340
Lee JM, Trevisani F, Vilgrain V, Wald C (2011) Imaging diagnosis and staging of hepatocellular carcinoma. Liver Transpl 17:S34–S43
Bruix J, Reig M, Rimola J, et al. (2011) Clinical decision making and research in hepatocellular carcinoma: pivotal role of imaging techniques. Hepatology 54:2238–2244
Ogawa S, Lee T-M, Kay AR, Tank DW (1990) Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci 87:9868–9872
Christen T, Lemasson B, Pannetier N, et al. (2012) Is T2* enough to assess oxygenation? Quantitative blood oxygen level–dependent analysis in brain tumor. Radiology 262:495–502
Pamela CC, Richard AH, Denise RF (2005) Lippincott’s illustrated reviews: biochemistry, 4th edn. Baltimore: Lippincott Williams & Wilkins, p 135
Koutcher JA, Alfieri AA, Devitt ML, et al. (1992) Quantitative changes in tumor metabolism, partial pressure of oxygen, and radiobiological oxygenation status postradiation. Can Res 52:4620–4627
Sasaya S, Yagi H, Yamaguchi M, et al. (2000) Liver function assessed by increased rate of portal venous blood flow after oral intake of glucose. Eur J Surg 166:112–118
Mitchell DG, Bruix J, Sherman M, et al. (2015) LI-RADS (liver imaging reporting and data system): summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology 61(3):1056–1065
Haque M, Koktzoglou I, Li W, Carbray J, Prasad P (2010) Functional MRI of liver using BOLD MRI: effect of glucose. J Magn Reson Imaging 32:988–991
Ludwig D, Schwarting K, Korbel CM, et al. (1998) The postprandial portal flow is related to the severity of portal hypertension and liver cirrhosis. J Hepatol 28:631–638
Joynt LK, Platt JF, Rubin JM, Ellis JH, Bude RO (1995) Hepatic artery resistance before and after standard meal in subjects with diseased and healthy livers. Radiology 196:489–492
Cheng HLM (2012) Effect of hyperoxia and hypercapnia on tissue oxygen and perfusion response in the normal liver and kidney. PLoS ONE 7:12
Barash H, Gross E, Matot I, et al. (2007) Functional mr imaging during hypercapnia and hyperoxia: noninvasive tool for monitoring changes in liver perfusion and hemodynamics in a rat model 1. Radiology 243:727–735
Patterson AJ, Priest AN, Bowden DJ, et al. (2016) Quantitative BOLD imaging at 3T: Temporal changes in hepatocellular carcinoma and fibrosis following oxygen challenge. J Magn Reson Imaging 44:739–744
Roldán-Alzate A, Frydrychowicz A, Said A, et al. (2015) Impaired regulation of portal venous flow in response to a meal challenge as quantified by 4D flow MRI. J Magn Reson Imaging 42:1009–1017
Tappy L, Schneiter P, Chioléro R, Bettschart V, Gillet M (2001) Effects of a glucose meal on energy metabolism in patients with cirrhosis before and after liver transplantation. Arch Surg 136:80–84
Iwakiri Y, Shah V, Rockey DC (2014) Vascular pathobiology in chronic liver disease and cirrhosis—current status and future directions. J Hepatol 61:912–924
Annet L, Materne R, Danse E, et al. (2003) Hepatic flow parameters measured with MR imaging and doppler US: correlations with degree of cirrhosis and portal hypertension 1. Radiology 229:409–414
Toyoda H, Fukuda Y, Hayakawa T, Kumada T, Nakano S (1997) Changes in blood supply in small hepatocellular carcinoma: correlation of angiographic images and immunohistochemical findings. J Hepatol 27:654–660
Hayashi M, Matsui O, Ueda K, et al. (2002) Progression to hypervascular hepatocellular carcinoma: correlation with Intranodular blood supply evaluated with CT during intraarterial injection of contrast material 1. Radiology 225:143–149
Okino Y, Kiyosue H, Matsumoto S, et al. (2003) Hepatocellular carcinoma: prediction of blood supply from right inferior phrenic artery by multiphasic CT. J Comput Assist Tomogr 27:341–346
Asayama Y, Yoshimitsu K, Nishihara Y, et al. (2008) Arterial blood supply of hepatocellular carcinoma and histologic grading: radiologic-pathologic correlation. Am J Roentgenol 190:W28–W34
Beyoğlu D, Idle JR (2013) The metabolomic window into hepatobiliary disease. J Hepatol 59:842–858
Lee M, Yoon J-H (2015) Metabolic interplay between glycolysis and mitochondrial oxidation: the reverse Warburg effect and its therapeutic implication. World J Biol Chem 6:148–161
Schalkx HJ, Petersen ET, Peters NH, et al. (2015) Arterial and portal venous liver perfusion using selective spin labelling MRI. Eur Radiol 25:1529–1540
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Yuan, F., Song, B., Huang, Z. et al. Glucose as a stimulation agent in the BOLD functional magnetic resonance imaging for liver cirrhosis and hepatocellular carcinoma: a feasibility study. Abdom Radiol 43, 607–612 (2018). https://doi.org/10.1007/s00261-017-1264-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-017-1264-7