Abstract
Purpose
To investigate diagnostic efficiency of DWI using entire-tumor histogram analysis in differentiating the low-grade (LG) prostate cancer (PCa) from intermediate–high-grade (HG) PCa in comparison with conventional ROI-based measurement.
Methods
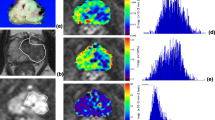
DW images (b of 0–1400 s/mm2) from 126 pathology-confirmed PCa (diameter >0.5 cm) in 110 patients were retrospectively collected and processed by mono-exponential model. The measurement of tumor apparent diffusion coefficients (ADCs) was performed with using histogram-based and ROI-based approach, respectively. The diagnostic ability of ADCs from two methods for differentiating LG-PCa (Gleason score, GS ≤6) from HG-PCa (GS > 6) was determined by ROC regression, and compared by McNemar’s test.
Results
There were 49 LG-tumor and 77 HG-tumor at pathologic findings. Histogram-based ADCs (mean, median, 10th and 90th) and ROI-based ADCs (mean) showed dominant relationships with ordinal GS of Pca (ρ = −0.225 to −0.406, p < 0.05). All above imaging indices reflected significant difference between LG-PCa and HG-PCa (all p values <0.01). Histogram 10th ADCs had dominantly high Az (0.738), Youden index (0.415), and positive likelihood ratio (LR+, 2.45) in stratifying tumor GS against mean, median and 90th ADCs, and ROI-based ADCs. Histogram mean, median, and 10th ADCs showed higher specificity (65.3%–74.1% vs. 44.9%, p < 0.01), but lower sensitivity (57.1%–71.3% vs. 84.4%, p < 0.05) than ROI-based ADCs in differentiating LG-PCa from HG-PCa.
Conclusions
DWI-associated histogram analysis had higher specificity, Az, Youden index, and LR+ for differentiation of PCa Gleason grade than ROI-based approach.




Similar content being viewed by others
References
Siegel R, Ma J, Zou Z, Jemal A (2014) Cancer statistics, 2014. CA Cancer J Clin 64(1):9–29
Wilt TJ, MacDonald R, Rutks I, et al. (2008) Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med 148(6):435–448
Grimm P, Billiet I, Bostwick D, et al. (2012) Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the Prostate Cancer Results Study Group. BJU Int 109(Suppl 1):22–29
D’Amico AV, Whittington R, Malkowicz SB, et al. (1998) Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 280(11):969–974
Namiki S, Saito S, Ishidoya S, et al. (2005) Adverse effect of radical prostatectomy on nocturia and voiding frequency symptoms. Urology 66(1):147–151
Yakar D, Debats OA, Bomers JG, et al. (2012) Predictive value of MRI in the localization, staging, volume estimation, assessment of aggressiveness, and guidance of radiotherapy and biopsies in prostate cancer. J Magn Reson Imaging 35(1):20–31
Tamada T, Sone T, Jo Y, et al. (2008) Apparent diffusion coefficient values in peripheral and transition zones of the prostate: comparison between normal and malignant prostatic tissues and correlation with histologic grade. J Magn Reson Imaging 28(3):720–726
Barentsz JO, Richenberg J, Clements R, et al. (2012) ESUR prostate MR guidelines 2012. Eur Radiol 22(4):746–757
Jung SI, Donati OF, Vargas HA, et al. (2013) Transition zone prostate cancer: incremental value of diffusion-weighted endorectal MR imaging in tumor detection and assessment of aggressiveness. Radiology 269(2):493–503
Vargas HA, Akin O, Franiel T, et al. (2011) Diffusion-weighted endorectal MR imaging at 3 T for prostate cancer: tumor detection and assessment of aggressiveness. Radiology 259(3):775–784
Nagarajan R, Margolis D, Raman S, et al. (2012) Correlation of Gleason scores with diffusion-weighted imaging findings of prostate cancer. Adv Urol 2012:374805
Turkbey B, Shah VP, Pang Y, et al. (2011) Is apparent diffusion coefficient associated with clinical risk scores for prostate cancers that are visible on 3-T MR images? Radiology 258(2):488–495
Hambrock T, Somford DM, Huisman HJ, et al. (2011) Relationship between apparent diffusion coefficients at 3.0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology 259(2):453–461
Wang H, Cheng L, Zhang X, et al. (2010) Renal cell carcinoma: diffusion-weighted MR imaging for subtype differentiation at 3.0 T. Radiology 257(1):135–143
Rosenkrantz AB, Niver BE, Fitzgerald EF, et al. (2010) Utility of the apparent diffusion coefficient for distinguishing clear cell renal cell carcinoma of low and high nuclear grade. AJR Am J Roentgenol 195(5):W344–W351
Lopez-Otin C, Diamandis EP (1998) Breast and prostate cancer: an analysis of common epidemiological, genetic, and biochemical features. Endocr Rev 19(4):365–396
Van der Kwast T, Bubendorf L, Mazerolles C, et al. (2013) Guidelines on processing and reporting of prostate biopsies: the 2013 update of the pathology committee of the European Randomized Study of Screening for Prostate Cancer (ERSPC). Virchows Arch 463(3):367–377
Woo S, Cho JY, Kim SY, Kim SH (2014) Histogram analysis of apparent diffusion coefficient map of diffusion-weighted MRI in endometrial cancer: a preliminary correlation study with histological grade. Acta Radiol 55(10):1270–1277
Kang Y, Choi SH, Kim YJ, et al. (2011) Gliomas: histogram analysis of apparent diffusion coefficient maps with standard- or high-b-value diffusion-weighted MR imaging—correlation with tumor grade. Radiology 261(3):882–890
Kim HS, Suh CH, Kim N, Choi CG, Kim SJ (2014) Histogram analysis of intravoxel incoherent motion for differentiating recurrent tumor from treatment effect in patients with glioblastoma: initial clinical experience. AJNR Am J Neuroradiol 35(3):490–497
Zhang YD, Wang Q, Wu CJ, et al. (2015) The histogram analysis of diffusion-weighted intravoxel incoherent motion (IVIM) imaging for differentiating the gleason grade of prostate cancer. Eur Radiol 25:994–1004
Peng Y, Jiang Y, Yang C, et al. (2013) Quantitative analysis of multiparametric prostate MR images: differentiation between prostate cancer and normal tissue and correlation with Gleason score—a computer-aided diagnosis development study. Radiology 267(3):787–796
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44(3):837–845
Donati OF, Mazaheri Y, Afaq A, et al. (2014) Prostate cancer aggressiveness: assessment with whole-lesion histogram analysis of the apparent diffusion coefficient. Radiology 271(1):143–152
Tozer DJ, Jager HR, Danchaivijitr N, et al. (2007) Apparent diffusion coefficient histograms may predict low-grade glioma subtype. NMR Biomed 20(1):49–57
Pope WB, Kim HJ, Huo J, et al. (2009) Recurrent glioblastoma multiforme: ADC histogram analysis predicts response to bevacizumab treatment. Radiology 252(1):182–189
Kyriazi S, Collins DJ, Messiou C, et al. (2011) Metastatic ovarian and primary peritoneal cancer: assessing chemotherapy response with diffusion-weighted MR imaging–value of histogram analysis of apparent diffusion coefficients. Radiology 261(1):182–192
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, CJ., Wang, Q., Li, H. et al. DWI-associated entire-tumor histogram analysis for the differentiation of low-grade prostate cancer from intermediate–high-grade prostate cancer. Abdom Imaging 40, 3214–3221 (2015). https://doi.org/10.1007/s00261-015-0499-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-015-0499-4