Abstract
Purpose
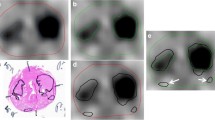
68 Ga-PSMA PET/CT has high specificity and sensitivity for the detection of both intraprostatic tumor focal lesions and metastasis. However, approximately 10% of primary prostate cancer are invisible on PSMA-PET (exhibit no or minimal uptake). In this work, we investigated whether machine learning-based radiomics models derived from PSMA-PET images could predict invisible intraprostatic lesions on 68 Ga-PSMA-11 PET in patients with primary prostate cancer.
Methods
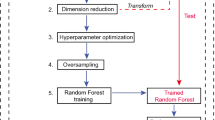
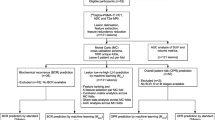
In this retrospective study, patients with or without prostate cancer who underwent 68 Ga-PSMA PET/CT and presented negative on PSMA-PET image at either of two different institutions were included: institution 1 (between 2017 and 2020) for the training set and institution 2 (between 2019 and 2020) for the external test set. Three random forest (RF) models were built using selected features extracted from standard PET images, delayed PET images, and both standard and delayed PET images. Then, subsequent tenfold cross-validation was performed. In the test phase, the three RF models and PSA density (PSAD, cut-off value: 0.15 ng/ml/ml) were tested with the external test set. The area under the receiver operating characteristic curve (AUC) was calculated for the models and PSAD. The AUCs of the radiomics model and PSAD were compared.
Results
A total of 64 patients (39 with prostate cancer and 25 with benign prostate disease) were in the training set, and 36 (21 with prostate cancer and 15 with benign prostate disease) were in the test set. The average AUCs of the three RF models from tenfold cross-validation were 0.87 (95% CI: 0.72, 1.00), 0.86 (95% CI: 0.63, 1.00), and 0.91 (95% CI: 0.69, 1.00), respectively. In the test set, the AUCs of the three trained RF models and PSAD were 0.903 (95% CI: 0.830, 0.975), 0.856 (95% CI: 0.748, 0.964), 0.925 (95% CI:0.838, 1.00), and 0.662 (95% CI: 0.510, 0.813). The AUCs of the three radiomics models were higher than that of PSAD (0.903, 0.856, and 0.925 vs. 0.662, respectively; P = .007, P = .045, and P = .005, respectively).
Conclusion
Random forest models developed by 68 Ga-PSMA-11 PET-based radiomics features were proven useful for accurate prediction of invisible intraprostatic lesion on 68 Ga-PSMA-11 PET in patients with primary prostate cancer and showed better diagnostic performance compared with PSAD.





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
The code applied during and/or analyzed during the current study is available from the corresponding author on reasonable request.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. https://doi.org/10.3322/caac.21551.
Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389:815–22. https://doi.org/10.1016/S0140-6736(16)32401-1.
Mottet N, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79:243–62. https://doi.org/10.1016/j.eururo.2020.09.042
Schouten MG, van der Leest M, Pokorny M, Hoogenboom M, Barentsz JO, Thompson LC, et al. Why and where do we miss significant prostate cancer with multi-parametric magnetic resonance imaging followed by magnetic resonance-guided and transrectal ultrasound-guided biopsy in biopsy-naive men? Eur Urol. 2017;71:896–903. https://doi.org/10.1016/j.eururo.2016.12.006.
Ukimura O, Coleman JA, de la Taille A, Emberton M, Epstein JI, Freedland SJ, et al. Contemporary role of systematic prostate biopsies: indications, techniques, and implications for patient care. Eur Urol. 2013;63:214–30. https://doi.org/10.1016/j.eururo.2012.09.033.
Abraham NE, Mendhiratta N, Taneja SS. Patterns of repeat prostate biopsy in contemporary clinical practice. J Urol. 2015;193:1178–84. https://doi.org/10.1016/j.juro.2014.10.084.
Le JD, Tan N, Shkolyar E, Lu DY, Kwan L, Marks LS, et al. Multifocality and prostate cancer detection by multiparametric magnetic resonance imaging: correlation with whole-mount histopathology. Eur Urol. 2015;67:569–76. https://doi.org/10.1016/j.eururo.2014.08.079.
Borofsky S, George AK, Gaur S, Bernardo M, Greer MD, Mertan FV, et al. What are we missing? False-negative cancers at multiparametric MR imaging of the prostate. Radiology. 2018;286:186–95. https://doi.org/10.1148/radiol.2017152877.
Panebianco V, Barchetti G, Simone G, Del Monte M, Ciardi A, Grompone MD, et al. Negative multiparametric magnetic resonance imaging for prostate cancer: what’s next? Eur Urol. 2018;74:48–54. https://doi.org/10.1016/j.eururo.2018.03.007.
Fendler WP, Calais J, Eiber M, Flavell RR, Mishoe A, Feng FY, et al. Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA Oncol. 2019;5:856–63. https://doi.org/10.1001/jamaoncol.2019.0096.
Donato P, Roberts MJ, Morton A, Kyle S, Coughlin G, Esler R, et al. Improved specificity with (68)Ga PSMA PET/CT to detect clinically significant lesions “invisible” on multiparametric MRI of the prostate: a single institution comparative analysis with radical prostatectomy histology. Eur J Nucl Med Mol Imaging. 2019;46:20–30. https://doi.org/10.1007/s00259-018-4160-7.
Donato P, Morton A, Yaxley J, Ranasinghe S, Teloken PE, Kyle S, et al. (68)Ga-PSMA PET/CT better characterises localised prostate cancer after MRI and transperineal prostate biopsy: is (68)Ga-PSMA PET/CT guided biopsy the future? Eur J Nucl Med Mol Imaging. 2020;47:1843–51. https://doi.org/10.1007/s00259-019-04620-0.
Roach PJ, Francis R, Emmett L, Hsiao E, Kneebone A, Hruby G, et al. The impact of (68)Ga-PSMA PET/CT on management intent in prostate cancer: results of an Australian prospective multicenter study. J Nucl Med. 2018;59:82–8. https://doi.org/10.2967/jnumed.117.197160.
Zhang LL, Li WC, Xu Z, Jiang N, Zang SM, Xu LW, et al. (68)Ga-PSMA PET/CT targeted biopsy for the diagnosis of clinically significant prostate cancer compared with transrectal ultrasound guided biopsy: a prospective randomized single-centre study. Eur J Nucl Med Mol Imaging. 2021;48:483–92. https://doi.org/10.1007/s00259-020-04863-2.
Zamboglou C, Bettermann AS, Gratzke C, Mix M, Ruf J, Kiefer S, et al. Uncovering the invisible-prevalence, characteristics, and radiomics feature-based detection of visually undetectable intraprostatic tumor lesions in (68)GaPSMA-11 PET images of patients with primary prostate cancer. Eur J Nucl Med Mol Imaging. 2021;48:1987–97. https://doi.org/10.1007/s00259-020-05111-3.
Zhang J, Shao S, Wu P, Liu D, Yang B, Han D, et al. Diagnostic performance of (68)Ga-PSMA PET/CT in the detection of prostate cancer prior to initial biopsy: comparison with cancer-predicting nomograms. Eur J Nucl Med Mol Imaging. 2019;46:908–20. https://doi.org/10.1007/s00259-018-4255-1.
Yaxley JW, Raveenthiran S, Nouhaud FX, Samaratunga H, Yaxley WJ, Coughlin G, et al. Risk of metastatic disease on (68) gallium-prostate-specific membrane antigen positron emission tomography/computed tomography scan for primary staging of 1253 men at the diagnosis of prostate cancer. BJU Int. 2019;124:401–7. https://doi.org/10.1111/bju.14828.
Uprimny C, Kroiss AS, Decristoforo C, Fritz J, von Guggenberg E, Kendler D, et al. (68)Ga-PSMA-11 PET/CT in primary staging of prostate cancer: PSA and Gleason score predict the intensity of tracer accumulation in the primary tumour. Eur J Nucl Med Mol Imaging. 2017;44:941–9. https://doi.org/10.1007/s00259-017-3631-6.
Maurer T, Gschwend JE, Rauscher I, Souvatzoglou M, Haller B, Weirich G, et al. Diagnostic efficacy of (68)Gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J Urol. 2016;195:1436–43. https://doi.org/10.1016/j.juro.2015.12.025.
Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2016;278:563–77. https://doi.org/10.1148/radiol.2015151169.
Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14:749–62. https://doi.org/10.1038/nrclinonc.2017.141.
Liu Z, Wang S, Dong D, Wei J, Fang C, Zhou X, et al. The applications of radiomics in precision diagnosis and treatment of oncology: opportunities and challenges. Theranostics. 2019;9:1303–22. https://doi.org/10.7150/thno.30309.
Wibmer A, Hricak H, Gondo T, Matsumoto K, Veeraraghavan H, Fehr D, et al. Haralick texture analysis of prostate MRI: utility for differentiating non-cancerous prostate from prostate cancer and differentiating prostate cancers with different Gleason scores. Eur Radiol. 2015;25:2840–50. https://doi.org/10.1007/s00330-015-3701-8.
Zamboglou C, Carles M, Fechter T, Kiefer S, Reichel K, Fassbender TF, et al. Radiomic features from PSMA PET for non-invasive intraprostatic tumor discrimination and characterization in patients with intermediate- and high-risk prostate cancer – a comparison study with histology reference. Theranostics. 2019;9:2595–605. https://doi.org/10.7150/thno.32376.
Bonekamp D, Kohl S, Wiesenfarth M, Schelb P, Radtke JP, Gotz M, et al. Radiomic machine learning for characterization of prostate lesions with mri: comparison to ADC values. Radiology. 2018;289:128–37. https://doi.org/10.1148/radiol.2018173064.
Gong L, Xu M, Fang M, Zou J, Yang S, Yu X, et al. Noninvasive prediction of high-grade prostate cancer via biparametric MRI radiomics. J Magn Reson Imaging. 2020;52:1102–9. https://doi.org/10.1002/jmri.27132.
Sun Y, Reynolds HM, Wraith D, Williams S, Finnegan ME, Mitchell C, et al. Automatic stratification of prostate tumour aggressiveness using multiparametric MRI: a horizontal comparison of texture features. Acta Oncol. 2019;58:1118–26. https://doi.org/10.1080/0284186X.2019.1598576.
Alongi P, Stefano A, Comelli A, Laudicella R, Scalisi S, Arnone G, et al. Radiomics analysis of 18F-Choline PET/CT in the prediction of disease outcome in high-risk prostate cancer: an explorative study on machine learning feature classification in 94 patients. Eur Radiol. 2021;31:4595–605. https://doi.org/10.1007/s00330-020-07617-8.
Rauscher I, Maurer T, Fendler WP, Sommer WH, Schwaiger M, Eiber M. (68)Ga-PSMA ligand PET/CT in patients with prostate cancer: how we review and report. Cancer Imaging. 2016;16:14. https://doi.org/10.1186/s40644-016-0072-6.
Yushkevich PA, Gerig G. ITK-SNAP: an intractive medical image segmentation tool to meet the need for expert-guided segmentation of complex medical images. IEEE Pulse. 2017;8:54–7. https://doi.org/10.1109/MPUL.2017.2701493.
Ding C, Peng H. Minimum redundancy feature selection from microarray gene expression data. J Bioinform Comput Biol. 2005;3:185–205. https://doi.org/10.1142/s0219720005001004.
Varkarakis I, Zarkadoulias A, Bourdoumis A, Chatzidarellis E, Antoniou N, Deliveliotis C. Measurement of PSA density by 3 imaging modalities and its correlation with the PSA density of radical prostatectomy specimen. Urol Oncol. 2013;31:1038–42. https://doi.org/10.1016/j.urolonc.2011.11.033.
Caglic I, Sushentsev N, Gnanapragasam VJ, Sala E, Shaida N, Koo BC, et al. MRI-derived PRECISE scores for predicting pathologically-confirmed radiological progression in prostate cancer patients on active surveillance. Eur Radiol. 2021;31:2696–705. https://doi.org/10.1007/s00330-020-07336-0.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45.
Xu M, Fang M, Zou J, Yang S, Yu D, Zhong L, et al. Using biparametric MRI radiomics signature to differentiate between benign and malignant prostate lesions. Eur J Radiol. 2019;114:38–44. https://doi.org/10.1016/j.ejrad.2019.02.032.
Hu Y, Zhao X, Zhang J, Han J, Dai M. Value of (18)F-FDG PET/CT radiomic features to distinguish solitary lung adenocarcinoma from tuberculosis. Eur J Nucl Med Mol Imaging. 2021;48:231–40. https://doi.org/10.1007/s00259-020-04924-6.
Bang JI, Ha S, Kang SB, Lee KW, Lee HS, Kim JS, et al. Prediction of neoadjuvant radiation chemotherapy response and survival using pretreatment [(18)F]FDG PET/CT scans in locally advanced rectal cancer. Eur J Nucl Med Mol Imaging. 2016;43:422–31. https://doi.org/10.1007/s00259-015-3180-9.
Chandarana H, Rosenkrantz AB, Mussi TC, Kim S, Ahmad AA, Raj SD, et al. Histogram analysis of whole-lesion enhancement in differentiating clear cell from papillary subtype of renal cell cancer. Radiology. 2012;265:790–8. https://doi.org/10.1148/radiol.12111281.
Khalvati F, Wong A, Haider MA. Automated prostate cancer detection via comprehensive multi-parametric magnetic resonance imaging texture feature models. BMC Med Imaging. 2015;15:27. https://doi.org/10.1186/s12880-015-0069-9.
Mohamed SS, Salama MM, Kamel M, El-Saadany EF, Rizkalla K, Chin J. Prostate cancer multi-feature analysis using trans-rectal ultrasound images. Phys Med Biol. 2005;50:N175–85. https://doi.org/10.1088/0031-9155/50/15/N02.
Falagario UG, Jambor I, Lantz A, Ettala O, Stabile A, Taimen P, et al. Combined use of prostate-specific antigen density and magnetic resonance imaging for prostate biopsy decision planning: a retrospective multi-institutional study using the Prostate Magnetic Resonance Imaging Outcome Database (PROMOD). Eur Urol Oncol. 2020. https://doi.org/10.1016/j.euo.2020.08.014.
Alberts AR, Roobol MJ, Drost FH, van Leenders GJ, Bokhorst LP, Bangma CH, et al. Risk-stratification based on magnetic resonance imaging and prostate-specific antigen density may reduce unnecessary follow-up biopsy procedures in men on active surveillance for low-risk prostate cancer. BJU Int. 2017;120:511–9. https://doi.org/10.1111/bju.13836.
Zheng XY, Xie LP, Wang YY, Ding W, Yang K, Shen HF, et al. The use of prostate specific antigen (PSA) density in detecting prostate cancer in Chinese men with PSA levels of 4–10 ng/mL. J Cancer Res Clin Oncol. 2008;134:1207–10. https://doi.org/10.1007/s00432-008-0400-8.
Bulman JC, Toth R, Patel AD, Bloch BN, McMahon CJ, Ngo L, et al. Automated computer-derived prostate volumes from MR imaging data: comparison with radiologist-derived MR imaging and pathologic specimen volumes. Radiology. 2012;262:144–51. https://doi.org/10.1148/radiol.11110266.
Acknowledgements
The authors would like to acknowledge all the co-workers who participated in this study.
Funding
This study was supported by the Medical Science and Technology Research Fund Project of Guangdong (A2019526) and the Guangzhou Science & Technology Project (201802020033).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. The research was designed by Ningyi Jiang and Yong Zhang. Material preparation, data collection, and analysis were performed by Zhilong Yi, Siqi Hu, Xiaofeng Lin, Qiong Zou, MinHong Zou, Zhanlei Zhang, and Lei Xu. The first draft of the manuscript was written by Zhilong Yi and reviewed by Ningyi Jiang and Yong Zhang. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This retrospective study was approved by the institutional review boards of the two hospitals, and the requirements to obtain informed consent were waived.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Advanced Image Analyses (Radiomics and Artificial Intelligence)
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yi, Z., Hu, S., Lin, X. et al. Machine learning-based prediction of invisible intraprostatic prostate cancer lesions on 68 Ga-PSMA-11 PET/CT in patients with primary prostate cancer. Eur J Nucl Med Mol Imaging 49, 1523–1534 (2022). https://doi.org/10.1007/s00259-021-05631-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-021-05631-6