Abstract
Purpose
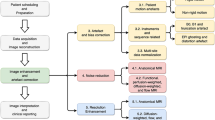
Deep convolutional neural networks (CNN) for single photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) has been used to improve the diagnostic accuracy of coronary artery disease (CAD). This study was to design and evaluate a deep learning (DL) approach to automatic diagnosis of myocardial perfusion abnormalities from stress-only MPI.
Methods
The new DL approach developed for this study was compared to a conventional quantitative perfusion defect size (DS) method. A total of 37,243 patients (51.5% males) undergone stress 99mTc-Tetrofosmin or 99mTc-Sestamibi MPI were selected retrospectively from Yale New Haven Hospital. Patients were dichotomized as studies with normal (75.4%) or abnormal (24.6%) myocardial perfusion based on final diagnoses of clinical nuclear cardiologists. Stress myocardial perfusion defect size was calculated using Yale quantitative analytic software. A deep CNN was trained using the circumferential count profile maps derived from SPECT MPI and was evaluated for the diagnosis of perfusion abnormality with a 5-fold cross-validation approach. In each fold, 27,933, 1862 and 7448 patients were used as training, validation and testing datasets, respectively. The area under the receiver-operating characteristic curve (AUC) was calculated and analyzed for all patients as well as for the eight sub-groups classified based on patient genders, quantitative algorithms, radioactive tracers and SPECT cameras.
Results
The AUC value resulted from the DL method was significantly higher than that from the DS method (0.872 ± 0.002 vs. 0.838 ± 0.003, p < 0.01). Across the eight sub-groups, the DL method provided more consistent AUC values in terms of smaller standard deviation and higher diagnostic accuracy and specificity, but slightly lower sensitivity than the DS method (AUC: 0.865 ± 0.010 vs. 0.838 ± 0.019, Accuracy: 82.7% ± 2.5% vs. 78.5% ± 3.6%, Specificity: 84.9% ± 3.7% vs. 77.5% ± 6.5%, Sensitivity: 74.4% ± 4.2% vs. 79.8% ± 5.8%).
Conclusions
The incorporation of deep learning for stress-only MPI has a considerable potential to improve the diagnostic accuracy and consistency in the detection of myocardial perfusion abnormalities.



Similar content being viewed by others
References
Hansen CL, Goldstein RA, Akinboboye OO, Berman DS, Botvinick EH, Churchwell KB, et al. Myocardial perfusion and function: single photon emission computed tomography. J Nucl Cardiol. 2007;14:e39–60.
Yokota S, Mouden M, Ottervanger JP, Engbers E, Knollema S, Timmer JR, et al. Prognostic value of normal stress-only myocardial perfusion imaging: a comparison between conventional and CZT-based SPECT. Eur J Nucl Med Mol Imaging. 2016;43:296–301.
Henzlova MJ, Duvall WL, Einstein AJ, Travin MI, Verberne HJ. ASNC imaging guidelines for SPECT nuclear cardiology procedures: stress, protocols, and tracers. J Nucl Cardiol. 2016;23:606–39.
Spadafora M, Salvatore M, Cuocolo A. Stress protocol and accuracy of myocardial perfusion imaging: is it better to start from the end? Springer; 2016.
Gowd BP, Heller GV, Parker MW. Stress-only SPECT myocardial perfusion imaging: a review. J Nucl Cardiol. 2014;21:1200–12.
Henzlova MJ, Croft LB, Duvall WL. Stress-only imaging: Faster, cheaper, less radiation. So what’s the hold up? : Springer; 2013.
Liu Y-H, Sinusas AJ, DeMan P, Zaret BL, Frans J. Quantification of SPECT myocardial perfusion images: methodology and validation of the Yale-CQ method. J Nucl Cardiol. 1999;6:190–203.
Slomka PJ, Nishina H, Berman DS, Akincioglu C, Abidov A, Friedman JD, et al. Automated quantification of myocardial perfusion SPECT using simplified normal limits. J Nucl Cardiol. 2005;12:66–77.
Liu Y-H. Quantification of nuclear cardiac images: the Yale approach. J Nucl Cardiol. 2007;14:483–91.
Nakanishi R, Gransar H, Slomka P, Arsanjani R, Shalev A, Otaki Y, et al. Predictors of high-risk coronary artery disease in subjects with normal SPECT myocardial perfusion imaging. J Nucl Cardiol. 2016;23:530–41. https://doi.org/10.1007/s12350-015-0150-3.
Slomka P, Hung G-U, Germano G, Berman DS. Novel SPECT technologies and approaches in cardiac imaging. Cardiovascular innovations and applications. 2016;2:31.
Rubeaux M, Xu Y, Germano G, Berman DS, Slomka PJ. Normal databases for the relative quantification of myocardial perfusion. Current cardiovascular imaging reports. 2016;9:22.
Dey D, Slomka PJ, Leeson P, Comaniciu D, Shrestha S, Sengupta PP, et al. Artificial intelligence in cardiovascular imaging: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:1317–35.
Al’Aref SJ, Anchouche K, Singh G, Slomka PJ, Kolli KK, Kumar A, et al. Clinical applications of machine learning in cardiovascular disease and its relevance to cardiac imaging. Eur Heart J. 2018;40:1975–86.
Gomez J, Doukky R, Germano G, Slomka P. New trends in quantitative nuclear cardiology methods. Current Cardiovascular Imaging Reports. 2018;11:1.
Nakajima K, Kudo T, Nakata T, Kiso K, Kasai T, Taniguchi Y, et al. Diagnostic accuracy of an artificial neural network compared with statistical quantitation of myocardial perfusion images: a Japanese multicenter study. Eur J Nucl Med Mol Imaging. 2017;44:2280–9.
Betancur J, Otaki Y, Motwani M, Fish MB, Lemley M, Dey D, et al. Prognostic value of combined clinical and myocardial perfusion imaging data using machine learning. JACC Cardiovasc Imaging. 2017;2406.
Shrestha S, Sengupta PP. Machine learning for nuclear cardiology: the way forward: Springer; 2018.
Alonso DH, Wernick MN, Yang Y, Germano G, Berman DS, Slomka P. Prediction of cardiac death after adenosine myocardial perfusion SPECT based on machine learning. J Nucl Cardiol. 2019;26:1746–54.
Arsanjani R, Dey D, Khachatryan T, Shalev A, Hayes SW, Fish M, et al. Prediction of revascularization after myocardial perfusion SPECT by machine learning in a large population. J Nucl Cardiol. 2015;22:877–84.
Chen X-W, Lin X. Big data deep learning: challenges and perspectives. IEEE access. 2014;2:514–25.
LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436–44. https://doi.org/10.1038/nature14539.
Henglin M, Stein G, Hushcha PV, Snoek J, Wiltschko AB, Cheng S. Machine learning approaches in cardiovascular imaging. Circulation: Cardiovascular Imaging. 2017;10:e005614.
Betancur J, Commandeur F, Motlagh M, Sharir T, Einstein AJ, Bokhari S, et al. Deep learning for prediction of obstructive disease from fast myocardial perfusion SPECT: a multicenter study. JACC Cardiovasc Imaging. 2018;11:1654–63.
He K, Zhang X, Ren S, Sun J. Deep residual learning for image recognition. Proceedings of the IEEE conference on computer vision and pattern recognition; 2016. p. 770–8.
Maas AL, Hannun AY, Ng AY. Rectifier nonlinearities improve neural network acoustic models. Proc icml; 2013. p. 3.
Paszke A, Gross S, Massa F, Lerer A, Bradbury J, Chanan G, et al. Pytorch: An imperative style, high-performance deep learning library. Advances in neural information processing systems; 2019. p. 8026–37.
Loshchilov I, Hutter F. Sgdr: Stochastic gradient descent with warm restarts. arXiv preprint arXiv:160803983. 2016.
Sun X, Xu W. Fast implementation of DeLong’s algorithm for comparing the areas under correlated receiver operating characteristic curves. IEEE Signal Processing Letters. 2014;21:1389–93.
Fluss R, Faraggi D, Reiser B. Estimation of the Youden index and its associated cutoff point. Biometrical Journal: Journal of Mathematical Methods in Biosciences. 2005;47:458–72.
Acknowledgments
This material was supported by the State of Connecticut under the Connecticut Bioscience Innovation Fund (16-00248, LIU). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the State of Connecticut or Connecticut Innovations, Inc. No potential conflicts of interest relevant to this work. The GPU card was supported by a grant from NVDIA.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
No potential conflicts of interest relevant to this work.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Yale Institutional Review Board protocol approval # 2000028863) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardiology.
Rights and permissions
About this article
Cite this article
Liu, H., Wu, J., Miller, E.J. et al. Diagnostic accuracy of stress-only myocardial perfusion SPECT improved by deep learning. Eur J Nucl Med Mol Imaging 48, 2793–2800 (2021). https://doi.org/10.1007/s00259-021-05202-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-021-05202-9