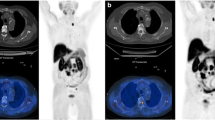
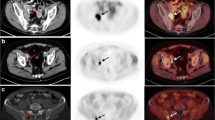
Abstract
Purpose
The aim of this study was to prospectively evaluate the value of [11C] Choline PET/CT in monitoring early and late response to a standardized first-line docetaxel chemotherapy in castration refractory prostate cancer (mCRPC) patients.
Methods
Thirty-two patients were referred for [11C] Choline PET/CT before the start of docetaxel chemotherapy, after one and ten chemotherapy cycles (or - in case of discontinuation - after the last administered cycle) for therapy response assessment. [11C] Choline uptake (SUVmax, SUVmean), CT derived Houndsfield units (HUmax, HUmean), and volume of bone, lung, and nodal metastases and local recurrence were measured semi-automatically at these timepoints. Change in SUVmax, SUVmean, HUmax, HUmean, and volume was assessed between PET 2 and 1 (early response assessment, ERA) and PET 3 and 1 (late response assessment, LRA) on a patient and lesion basis. Results of PET/CT were compared to clinically used RECIST 1.1 and clinical criteria based therapy response assessment including PSA for defining progressive disease (PD) and non-progressive disease (nPD), respectively. Relationships between changes of SUVmax and SUVmean (early and late) and changes of PSAearly and PSAlate were evaluated. Prognostic value of initial SUVmax and SUVmean was assessed. Statistical analyses were performed using SPSS.
Results
In the patient-based ERA and LRA there were no statistically significant differences in change of choline uptake, HU, and volume between PD and nPD applying RECIST or clinical response criteria. In the lesion-based ERA, decrease in choline uptake of bone metastases was even higher in PD (applying RECIST criteria), whereas in LRA the decrease was higher in nPD (applying clinical criteria). There were only significant correlations between change in choline uptake and PSA in ERA in PD, in LRA no significant correlations were discovered. Initial SUVmax and SUVmean were statistically significantly higher in nPD (applying clinical criteria).
Conclusion
There is no significant correlation between change in choline uptake in [11C] Choline PET/CT and clinically routinely used objective response assessment during the early and late course of docetaxel chemotherapy. Therefore, [11C] Choline PET/CT seems to be of limited use in therapy response assessment in standardized first-line chemotherapy in mCRPC patients.

Similar content being viewed by others
References
Siegel RL, Sahar L, Portier KM, Ward EM, Jemal A. Cancer death rates in US congressional districts. CA Cancer J Clin. 2015;65:339–44.
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65:467–79.
Berthold DR, Pond GR, Roessner M, de Wit R, Eisenberger M, Tannock AI. Treatment of hormone-refractory prostate cancer with docetaxel or mitoxantrone: relationships between prostate-specific antigen, pain, and quality of life response and survival in the TAX-327 study. Clin Cancer Res. 2008;14:2763–7.
Nakano K, Komatsu K, Kubo T, Natsui S, Nukui A, Kurokawa S, et al. External validation of risk classification in patients with docetaxel-treated castration-resistant prostate cancer. BMC Urol. 2014;14:31.
Thalgott M, Rack B, Eiber M, Souvatzoglou M, Heck MM, Kronester C, et al. Categorical versus continuous circulating tumor cell enumeration as early surrogate marker for therapy response and prognosis during docetaxel therapy in metastatic prostate cancer patients. BMC Cancer. 2015;15:458.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Messiou C, Cook G, De Souza NM. Imaging metastatic bone disease from carcinoma of the prostate. Br J Cancer. 2009;101:1225–32.
Bubley GJ, Carducci M, Dahut W, Dawson N, Daliani D, Eisenberger M, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the prostate-specific antigen working group. J Clin Oncol. 1999;17:3461–7.
Ceci F, Castellucci P, Graziani T, Schiavina R, Renzi R, Borghesi M, et al. (11) C-Choline PET/CT in castration-resistant prostate cancer patients treated with docetaxel. Eur J Nucl Med Mol Imaging. 2016;43:84–91.
Mulders PF, Schalken JA. Measuring therapeutic efficacy in the changing paradigm of castrate-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2009;12:241–6.
Basu S, Kumar R, Ranade R. Assessment of treatment response using PET. PET Clin. 2015;10:9–26.
Maffione AM, Marzola MC, Capirci C, Colletti PM, Rubello D. Value of (18) F-FDG PET for predicting response to Neoadjuvant therapy in rectal cancer: systematic review and meta-analysis. AJR Am J Roentgenol. 2015;204:1261–8.
Courtney KD, Manola JB, Elfiky AA, Ross R, Oh WK, Yap JT, et al. A phase I study of everolimus and docetaxel in patients with castration-resistant prostate cancer. Clin Genitourin Cancer. 2015;13:113–23.
De Giorgi U, Caroli P, Burgio SL, Menna C, Conteduca V, Bianchi E, et al. Early outcome prediction on 18F-fluorocholine PET/CT in metastatic castration-resistant prostate cancer patients treated with abiraterone. Oncotarget. 2014;5:12448–58.
De Giorgi U, Caroli P, Scarpi E, Conteduca V, Burgio SL, Menna C, et al. (18) F-Fluorocholine PET/CT for early response assessment in patients with metastatic castration-resistant prostate cancer treated with enzalutamide. Eur J Nucl Med Mol Imaging. 2015;42:1276–83.
Pascali C, Bogni A, Iwata R, Cambie M, Bombardieri E. [11C] methylation on a C18 Sep-Pak cartridge: a convenient way to produce [M-methyl-11C]choline. J Label Compd Radiopharm. 2000;43:195–203.
Khoury JD, Adcock DM, Chan F, Symanowski JT, Tiefenbacher S, Goodman O, et al. Increases in quantitative D-dimer levels correlate with progressive disease better than circulating tumor cell counts in patients with refractory prostate cancer. Am J Clin Pathol. 2010;134:964–9.
Thalgott M, Heck MM, Eiber M, Souvatzoglou M, Hatzichristodoulou G, Kehl V, et al. Circulating tumor cells versus objective response assessment predicting survival in metastatic castration-resistant prostate cancer patients treated with docetaxel chemotherapy. J Cancer Res Clin Oncol. 2015;141:1457–64.
Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European organization for research and treatment of cancer (EORTC) PET study group. Eur J Cancer. 1999;35:1773–82.
Evangelista L, Bombardieri E. Prostate-specific antigen and radiolabelled choline PET/CT for the assessment of response to therapy: synergy or conflicting? Eur J Nucl Med Mol Imaging. 2015
Caffo O, Maines F, Donner D, Veccia A, Chierichetti F, Galligioni E. Impact of enzalutamide administration on primary prostate cancer volume: a metabolic evaluation by choline positron emission tomography in castration-resistant prostate cancer patients. Clin Genitourin Cancer. 2014;12:312–6.
Pezaro CJ, Omlin A, Lorente D, Nava Rodrigues D, Ferraldeschi R, Bianchini D, et al. Visceral disease in castration-resistant prostate cancer. Eur Urol. 2014;65:270–3.
Oyama N, Akino H, Suzuki Y, Kanamaru H, Sadato N, Yonekura Y, et al. The increased accumulation of [18F] fluorodeoxyglucose in untreated prostate cancer. Jpn J Clin Oncol. 1999;29:623–9.
Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the prostate cancer clinical trials working group. J Clin Oncol. 2008;26:1148–59.
Acknowledgments
We thank Michael Souvatzoglou as well as the PET team, especially Brigitte Dzewas, Coletta Kruschke, and Nicola Henke.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Mark Thalgott and Bernd J. Krause shared senior authorship
Mark Thalgott and Bernd J. Krause contributed equally to this work.
Rights and permissions
About this article
Cite this article
Schwarzenböck, S.M., Eiber, M., Kundt, G. et al. Prospective evaluation of [11C]Choline PET/CT in therapy response assessment of standardized docetaxel first-line chemotherapy in patients with advanced castration refractory prostate cancer. Eur J Nucl Med Mol Imaging 43, 2105–2113 (2016). https://doi.org/10.1007/s00259-016-3439-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-016-3439-9