Abstract
Purpose
Quantitative estimates of dopamine transporter availability, determined with [123I]FP-CIT SPECT, depend on the SPECT equipment, including both hardware and (reconstruction) software, which limits their use in multicentre research and clinical routine. This study tested a dedicated reconstruction algorithm for its ability to reduce camera-specific intersubject variability in [123I]FP-CIT SPECT. The secondary aim was to evaluate binding in whole brain (excluding striatum) as a reference for quantitative analysis.
Methods
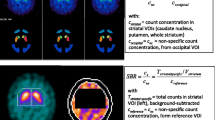
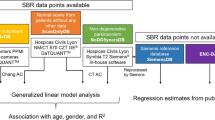
Of 73 healthy subjects from the European Normal Control Database of [123I]FP-CIT recruited at six centres, 70 aged between 20 and 82 years were included. SPECT images were reconstructed using the QSPECT software package which provides fully automated detection of the outer contour of the head, camera-specific correction for scatter and septal penetration by transmission-dependent convolution subtraction, iterative OSEM reconstruction including attenuation correction, and camera-specific “to kBq/ml” calibration. LINK and HERMES reconstruction were used for head-to-head comparison. The specific striatal [123I]FP-CIT binding ratio (SBR) was computed using the Southampton method with binding in the whole brain, occipital cortex or cerebellum as the reference. The correlation between SBR and age was used as the primary quality measure.
Results
The fraction of SBR variability explained by age was highest (1) with QSPECT, independently of the reference region, and (2) with whole brain as the reference, independently of the reconstruction algorithm.
Conclusion
QSPECT reconstruction appears to be useful for reduction of camera-specific intersubject variability of [123I]FP-CIT SPECT in multisite and single-site multicamera settings. Whole brain excluding striatal binding as the reference provides more stable quantitative estimates than occipital or cerebellar binding.






Similar content being viewed by others
References
Booij J, Speelman JD, Horstink MW, Wolters EC. The clinical benefit of imaging striatal dopamine transporters with [123I]FP-CIT SPET in differentiating patients with presynaptic parkinsonism from those with other forms of parkinsonism. Eur J Nucl Med. 2001;28:266–72.
Booij J, Tissingh G, Boer GJ, Speelman JD, Stoof JC, Janssen AG, et al. [123I]FP-CIT SPECT shows a pronounced decline of striatal dopamine transporter labelling in early and advanced Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1997;62:133–40.
Tatsch K, Poepperl G. Nigrostriatal dopamine terminal imaging with dopamine transporter SPECT: an update. J Nucl Med. 2013;54:1331–8. doi:10.2967/jnumed.112.105379.
Van Laere K, Everaert L, Annemans L, Gonce M, Vandenberghe W, Vander Borght T. The cost effectiveness of 123I-FP-CIT SPECT imaging in patients with an uncertain clinical diagnosis of parkinsonism. Eur J Nucl Med Mol Imaging. 2008;35:1367–76. doi:10.1007/s00259-008-0777-2.
Walker Z, Costa DC, Walker RW, Shaw K, Gacinovic S, Stevens T, et al. Differentiation of dementia with Lewy bodies from Alzheimer’s disease using a dopaminergic presynaptic ligand. J Neurol Neurosurg Psychiatry. 2002;73:134–40.
Booij J, Habraken JB, Bergmans P, Tissingh G, Winogrodzka A, Wolters EC, et al. Imaging of dopamine transporters with iodine-123-FP-CIT SPECT in healthy controls and patients with Parkinson’s disease. J Nucl Med. 1998;39:1879–84.
Darcourt J, Booij J, Tatsch K, Varrone A, Vander Borght T, Kapucu OL, et al. EANM procedure guidelines for brain neurotransmission SPECT using (123)I-labelled dopamine transporter ligands, version 2. Eur J Nucl Med Mol Imaging. 2010;37:443–50. doi:10.1007/s00259-009-1267-x.
Koch W, Radau PE, Hamann C, Tatsch K. Clinical testing of an optimized software solution for an automated, observer-independent evaluation of dopamine transporter SPECT studies. J Nucl Med. 2005;46:1109–18.
Soderlund TA, Dickson JC, Prvulovich E, Ben-Haim S, Kemp P, Booij J, et al. Value of semiquantitative analysis for clinical reporting of 123I-2-beta-carbomethoxy-3beta-(4-iodophenyl)-N-(3-fluoropropyl)nortropane SPECT studies. J Nucl Med. 2013;54:714–22. doi:10.2967/jnumed.112.110106.
Tatsch K, Poepperl G. Quantitative approaches to dopaminergic brain imaging. Q J Nucl Med Mol Imaging. 2012;56:27–38.
Tossici-Bolt L, Hoffmann SM, Kemp PM, Mehta RL, Fleming JS. Quantification of [123I]FP-CIT SPECT brain images: an accurate technique for measurement of the specific binding ratio. Eur J Nucl Med Mol Imaging. 2006;33:1491–9. doi:10.1007/s00259-006-0155-x.
Zubal IG, Early M, Yuan O, Jennings D, Marek K, Seibyl JP. Optimized, automated striatal uptake analysis applied to SPECT brain scans of Parkinson’s disease patients. J Nucl Med. 2007;48:857–64. doi:10.2967/jnumed.106.037432.
O’Brien JT, Colloby S, Fenwick J, Williams ED, Firbank M, Burn D, et al. Dopamine transporter loss visualized with FP-CIT SPECT in the differential diagnosis of dementia with Lewy bodies. Arch Neurol. 2004;61:919–25. doi:10.1001/archneur.61.6.919.
Graham LS, Fahey FH, Madsen MT, van Aswegen A, Yester MV. Quantitation of SPECT performance: Report of Task Group 4, Nuclear Medicine Committee. Med Phys. 1995;22:401–9.
Tossici-Bolt L, Dickson JC, Sera T, de Nijs R, Bagnara MC, Jonsson C, et al. Calibration of gamma camera systems for a multicentre European 123I-FP-CIT SPECT normal database. Eur J Nucl Med Mol Imaging. 2011;38:1529–40. doi:10.1007/s00259-011-1801-5.
McKeith I, O’Brien J, Walker Z, Tatsch K, Booij J, Darcourt J, et al. Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. Lancet Neurol. 2007;6:305–13. doi:10.1016/S1474-4422(07)70057-1.
Varrone A, Dickson JC, Tossici-Bolt L, Sera T, Asenbaum S, Booij J, et al. European multicentre database of healthy controls for [123I]FP-CIT SPECT (ENC-DAT): age-related effects, gender differences and evaluation of different methods of analysis. Eur J Nucl Med Mol Imaging. 2013;40:213–27. doi:10.1007/s00259-012-2276-8.
Nobili F, Naseri M, De Carli F, Asenbaum S, Booij J, Darcourt J, et al. Automatic semi-quantification of [123I]FP-CIT SPECT scans in healthy volunteers using BasGan version 2: results from the ENC-DAT database. Eur J Nucl Med Mol Imaging. 2013;40:565–73. doi:10.1007/s00259-012-2304-8.
Iida H, Eberl S. Quantitative assessment of regional myocardial blood flow with thallium-201 and SPECT. J Nucl Cardiol. 1998;5:313–31.
Iida H, Narita Y, Kado H, Kashikura A, Sugawara S, Shoji Y, et al. Effects of scatter and attenuation correction on quantitative assessment of regional cerebral blood flow with SPECT. J Nucl Med. 1998;39:181–9.
Kim K, Watabe H, Shidahara M, Ishida Y, Iida H. SPECT collimator dependency of scatter and validation of transmission-dependent scatter compensation methodologies. IEEE Trans Nucl Sci. 2001;48:689–96.
Iida H, Nakagawara J, Hayashida K, Fukushima K, Watabe H, Koshino K, et al. Multicenter evaluation of a standardized protocol for rest and acetazolamide cerebral blood flow assessment using a quantitative SPECT reconstruction program and split-dose 123I-iodoamphetamine. J Nucl Med. 2010;51:1624–31. doi:10.2967/jnumed.110.078352.
Yoneda H, Shirao S, Koizumi H, Oka F, Ishihara H, Ichiro K, et al. Reproducibility of cerebral blood flow assessment using a quantitative SPECT reconstruction program and split-dose 123I-iodoamphetamine in institutions with different γ-cameras and collimators. J Cereb Blood Flow Metab. 2012;32:1757–64. doi:10.1038/jcbfm.2012.67.
Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113.
Ichihara T, Ogawa K, Motomura N, Kubo A, Hashimoto S. Compton scatter compensation using the triple-energy window method for single- and dual-isotope SPECT. J Nucl Med. 1993;34:2216–21.
Ogawa K, Harata Y, Ichihara T, Kubo A, Hashimoto S. A practical method for position-dependent Compton-scatter correction in single photon emission CT. IEEE Trans Med Imaging. 1991;10:408–12. doi:10.1109/42.97591.
Hudson HM, Larkin RS. Accelerated image reconstruction using ordered subsets of projection data. IEEE Trans Med Imaging. 1994;13:601–9.
Meikle SR, Hutton BF, Bailey DL. A transmission-dependent method for scatter correction in SPECT. J Nucl Med. 1994;35:360–7.
Narita Y, Eberl S, Iida H, Hutton BF, Braun M, Nakamura T, et al. Monte Carlo and experimental evaluation of accuracy and noise properties of two scatter correction methods for SPECT. Phys Med Biol. 1996;41:2481–96.
Iida H, Shoji Y, Sugawara S, Kinoshita T, Tamura Y, Narita Y, et al. Design and experimental validation of a quantitative myocardial 201Tl SPECT system. IEEE Trans Nucl Sci. 1999;46:720–6.
Fleming JS, Bolt L, Stratford JS, Kemp PM. The specific uptake size index for quantifying radiopharmaceutical uptake. Phys Med Biol. 2004;49:N227–34.
Iida H, Hori Y, Ishida K, Imabayashi E, Matsuda H, Takahashi M, et al. Three-dimensional brain phantom containing bone and grey matter structures with a realistic head contour. Ann Nucl Med. 2013;27:25–36. doi:10.1007/s12149-012-0655-7.
Armitage P, Berry G. Statistical methods in medical research. 3rd ed. Oxford: Blackwell Science; 1994.
Hall H, Halldin C, Guilloteau D, Chalon S, Emond P, Besnard J, et al. Visualization of the dopamine transporter in the human brain postmortem with the new selective ligand [125I]PE2I. Neuroimage. 1999;9:108–16.
Djang DS, Janssen MJ, Bohnen N, Booij J, Henderson TA, Herholz K, et al. SNM practice guideline for dopamine transporter imaging with 123I-ioflupane SPECT 1.0. J Nucl Med. 2012;53:154–63. doi:10.2967/jnumed.111.100784.
Kupitz D, Apostolova I, Lange C, Ulrich G, Amthauer H, Brenner W, et al. Global scaling for semi-quantitative analysis in FP-CIT SPECT. Nuklearmedizin. 2014;53:234–41. doi:10.3413/Nukmed-0659-14-04.
Authors’ contributions
All authors made substantial contributions to the conception of the study, analysis of the data, and/or interpretation of the results. R.B. drafted the manuscript, and all other authors revised it critically for important intellectual content. All authors read and approved the final manuscript.
Compliance with ethical standards
ᅟ
Conflicts of interest
R.B. received an honorarium from ABX-CRO for independent statistical analysis, interpretation of results, and drafting the manuscript. ABX-CRO collaborates in development and distribution of the QSPECT software package, developed by H.I. J.B. is a consultant at GE Healthcare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
As the present study was retrospective in nature, formal consent was not required.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ralph Buchert and Andreas Kluge contributed equally to this work.
Rights and permissions
About this article
Cite this article
Buchert, R., Kluge, A., Tossici-Bolt, L. et al. Reduction in camera-specific variability in [123I]FP-CIT SPECT outcome measures by image reconstruction optimized for multisite settings: impact on age-dependence of the specific binding ratio in the ENC-DAT database of healthy controls. Eur J Nucl Med Mol Imaging 43, 1323–1336 (2016). https://doi.org/10.1007/s00259-016-3309-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-016-3309-5