Abstract
Purpose
We investigated the prognostic role of 68Ga-DOTANOC in patients affected by hepatic metastases from neuroendocrine tumours (NET) undergoing 90Y radioembolization (90Y-RE).
Methods
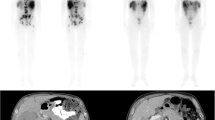
A group of 15 consecutive patients with unresectable NET liver metastases underwent 68Ga-DOTANOC PET at baseline and 6 weeks after 90Y-RE. Molecular response was defined as a reduction of >50 % in the tumour-to-spleen ratio (ΔT/S). The patients were divided into two groups (responders with ΔT/S >50 % and nonresponders with ΔT/S <50 %) Patients were followed up by imaging and laboratory tests every 3 months until death or for at least 36 months following 90Y-RE. Statistical analysis was performed to identify factors predicting overall survival (OS) and progression-free survival (PFS).
Results
A decrease in T/S ratio was seen in all patients on 68Ga-DOTANOC PET scans performed after 90Y-RE. Nine patients were classified as responders and six as nonresponders. The mean OS in all patients was 31.0 months. Responders had a significantly (p < 0.001) longer OS (mean 36.0 ± 2.5 months) and PFS (mean 29.7 ± 3.4 months) than nonresponders. In a multivariate analysis, none of the other examined variables including age, unilobar vs. bilobar locations, bilirubin levels, radiological response or the presence of extrahepatic disease significantly predicted patient outcome.
Conclusion
Molecular response assessed with 68Ga-DOTANOC PET might be a useful predictor of survival in patients affected by NET liver metastases treated with 90Y-RE.



Similar content being viewed by others
References
Zuetenhorst JM, Taal BG. Metastatic carcinoid tumors: a clinical review. Oncologist. 2005;10:123–31.
Basuroy R, Srirajaskanthan R, Ramage JK. A multimodal approach to the management of neuroendocrine tumour liver metastases. Int J Hepatol. 2012;2012:819193. doi:10.1155/2012/819193.
O’Toole D, Ruszniewski P. Chemoembolization and other ablative therapies for liver metastases of gastrointestinal endocrine tumours. Best Pract Res Clin Gastroenterol. 2005;19:585–94.
Van Essen M, Krenning EP, Kam BL, de Jong M, Valkema R, Kwekkeboom DJ. Peptide-receptor radionuclide therapy for endocrine tumors. Nat Rev Endocrinol. 2009;5:382–93.
McStay MK, Maudgil D, Williams M, Tibballs JM, Watkinson AF, Caplin ME, et al. Large-volume liver metastases from neuroendocrine tumors: hepatic intraarterial 90Y-DOTA-lanreotide as effective palliative therapy. Radiology. 2005;237:718–26.
Cianni R, Pelle G, Notarianni E, Saltarelli A, Rabuffi P, Bagni O, et al. Radioembolisation with (90)Y-labelled resin microspheres in the treatment of liver metastasis from breast cancer. Eur Radiol. 2013;23:182–9.
Rajekar H, Bogammana K, Stubbs RS. Selective internal radiation therapy for gastrointestinal neuroendocrine tumour liver metastases: a new and effective modality for treatment. Int J Hepatol. 2011;2011:404916. doi:10.4061/2011/404916.
Annunziata S, Treglia G, Caldarella C, Galiandro F. The role of 18F-FDG-PET and PET/CT in patients with colorectal liver metastases undergoing selective internal radiation therapy with yttrium-90: a first evidence-based review. ScientificWorldJournal. 2014;2014:879469. doi:10.1155/2014/879469.
Belhocine T, Foidart J, Rigo P, Najjar F, Thiry A, Quatresooz P, et al. Fluorodeoxyglucose positron emission tomography and somatostatin receptor scintigraphy for diagnosing and staging carcinoid tumors: correlations with the pathological indexes p53 and Ki-67. Nucl Med Commun. 2002;23:727–34.
Ambrosini V, Campana D, Tomassetti P, Fanti S. 68Ga-labelled peptides for diagnosis of gastroenteropancreatic NET. Eur J Nucl Med Mol Imaging. 2012;39:S52–60.
Koch W, Auernhammer CJ, Geisler J, Spitzweg C, Cyran CC, Ilhan H, et al. Treatment with octreotide in patients with well-differentiated neuroendocrine tumors of the ileum: prognostic stratification with Ga-68-DOTA-TATE positron emission tomography. Mol Imaging. 2014;13:1–10.
Kennedy A, Nag S, Salem R, Murthy R, McEwan AJ, Nutting C, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys. 2007;68:13–23.
Shaheen M, Hassanain M, Aljiffry M, Cabrera T, Chaudhury P, Simoneau E, et al. Predictors of response to radio-embolization (TheraSphere®) treatment of neuroendocrine liver metastasis. HPB (Oxford). 2012;14:60–6.
Bagni O, D'Arienzo M, Chiaramida P, Chiacchiararelli L, Cannas P, D’Agostini A, et al. 90Y-PET for the assessment of microsphere biodistribution after selective internal radiotherapy. Nucl Med Commun. 2012;33:198–204.
King J, Quinn R, Glenn DM, Janssen J, Tong D, Liaw W, et al. Radioembolization with selective internal radiation microspheres for neuroendocrine liver metastases. Cancer. 2008;113:921–9.
Frilling A, Modlin IM, Kidd M, Russell C, Breitenstein S, Salem R, et al. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol. 2014;15:e8–21.
Kennedy AS, Dezarn WA, McNeillie P, Coldwell D, Nutting C, Carter D, et al. Radioembolization for unresectable neuroendocrine hepatic metastases using resin 90Y-microspheres: early results in 148 patients. Am J Clin Oncol. 2008;31:271–9.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Barnacle AM, McHugh K. Limitations with the Response Evaluation Criteria in Solid Tumors (RECIST) guidance in disseminated pediatric malignancy. Pediatr Blood Cancer. 2006;46:127–34.
Peker A, Çiçek O, Soydal Ç, Küçük NÖ, Bilgiç S. Radioembolization with yttrium-90 resin microspheres for neuroendocrine tumor liver metastases. Diagn Interv Radiol. 2015;21:54–9.
Herder WW, Kwekkeboom DJ, Valkema R, Feelders RA, van Aken MO, Lamberts SW, et al. Neuroendocrine tumors and somatostatin: imaging techniques. J Endocrinol Invest. 2005;28:132–6.
Jamar F, Fiasse R, Leners N, Pauwels S. Somatostatin receptor imaging with indium-111-pentetreotide in gastroenteropancreatic neuroendocrine tumors: safety, efficacy and impact on patient management. J Nucl Med. 1995;36:542–9.
Kjaer A, Knigge U. Use of radioactive substances in diagnosis and treatment of neuroendocrine tumors. Scand J Gastroenterol. 2015;50:740–7.
Antunes P, Ginj M, Zhang H, Waser B, Baum RP, Reubi JC, et al. Are radiogallium-labelled DOTA-conjugated somatostatin analogues superior to those labelled with other radiometals? Eur J Nucl Med Mol Imaging. 2007;34:982–93.
Hofman MS, Kong G, Neels OC, Eu P, Hong E, Hicks RJ. High management impact of Ga-68 DOTATATE (GaTate) PET/CT for imaging neuroendocrine and other somatostatin expressing tumours. J Med Imaging Radiat Oncol. 2012;56:40–7.
Kabasakal L, Demirci E, Ocak M, Decristoforo C, Araman A, Ozsoy Y, et al. Comparison of 68Ga-DOTATATE and 68Ga-DOTANOC PET/CT imaging in the same patient group with neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2012;39:1271–7.
Sharma P, Arora S, Dhull VS, Naswa N, Kumar R, Ammini AC, et al. Evaluation of (68)Ga-DOTANOC PET/CT imaging in a large exclusive population of pancreatic neuroendocrine tumors. Abdom Imaging. 2015;40:299–309.
Ezziddin S, Meyer C, Kahancova S, Haslerud T, Willinek W, Wilhelm K, et al. 90Y Radioembolization after radiation exposure from peptide receptor radionuclide therapy. J Nucl Med. 2012;53:1663–9.
Gabriel M, Oberauer A, Dobrozemsky G, Decristoforo C, Putzer D, Kendler D, et al. 68Ga-DOTA-Tyr3-octreotide PET for assessing response to somatostatin-receptor-mediated radionuclide therapy. J Nucl Med. 2009;50:1427–34.
Kwee TC, Cheng G, Lam MG, Basu S, Alavi A. SUVmax of 2.5 should not be embraced as a magic threshold for separating benign from malignant lesions. Eur J Nucl Med Mol Imaging. 2013;40:1475–7.
Haug AR, Auernhammer CJ, Wängler B, Schmidt GP, Uebleis C, Göke B, et al. 68Ga-DOTATATE PET/CT for the early prediction of response to somatostatin receptor-mediated radionuclide therapy in patients with well-differentiated neuroendocrine tumors. J Nucl Med. 2010;5:1349–56.
Kratochwil C, Stefanova M, Mavriopoulou E, Holland-Letz T, Dimitrakopoulou-Strauss A, Afshar-Oromieh A, et al. SUV of 68GaDOTATOC-PET/CT predicts response probability of PRRT in neuroendocrine tumors. Mol Imaging Biol. 2015;17:313–8.
Ambrosini V, Tomassetti P, Castellucci P, Campana D, Montini G, Rubello D, et al. Comparison between 68Ga-DOTA-NOC and 18F-DOPA PET for the detection of gastro-entero-pancreatic and lung neuro-endocrine tumours. Eur J Nucl Med Mol Imaging. 2008;35:1431–8.
Bagni O, Filippi L, Schillaci O. 18F-FDG PET-derived parameters as prognostic indices in hepatic malignancies after 90Y radioembolization: is there a role? Eur J Nucl Med Mol Imaging. 2015;42:367–9.
Luboldt W, Hartmann H, Wiedemann B, Zöphel K, Luboldt HJ. Gastroenteropancreatic neuroendocrine tumors: standardizing therapy monitoring with 68Ga-DOTATOC PET/CT using the example of somatostatin receptor radionuclide therapy. Mol Imaging. 2010;9:351–8.
Compliance with ethical standards
Funding
None.
Conflicts of interest
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Filippi, L., Scopinaro, F., Pelle, G. et al. Molecular response assessed by 68Ga-DOTANOC and survival after 90Y microsphere therapy in patients with liver metastases from neuroendocrine tumours. Eur J Nucl Med Mol Imaging 43, 432–440 (2016). https://doi.org/10.1007/s00259-015-3178-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-015-3178-3