Abstract
Purpose
To assess correlations between the degree of dopaminergic depletion measured using single-photon emission computed tomography (SPECT) and different clinical parameters of disease progression in Parkinson’s disease (PD).
Methods
This retrospective study included 970 consecutive patients undergoing 123I-ioflupane SPECT scans in our institution between 2003 and 2013, from which we selected a study population of 411 patients according to their clinical diagnosis: 301 patients with PD (69.4 ± 11.0 years, of age, 163 men) and 110 patients with nondegenerative conditions included as controls (72.7 ± 8.0 years of age, 55 men). Comprehensive and operator-independent data analysis included spatial normalization into standard space, estimation of the mean uptake values in the striatum (caudate nucleus + putamen) and voxel-wise correlation between SPECT signal intensity and disease stage as well as disease duration in order to investigate the spatiotemporal pattern of the dopaminergic nigrostriatal degeneration. To compensate for potential interactions between disease stage and disease duration, one parameter was used as nonexplanatory coregressor for the other.
Results
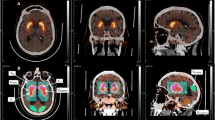
Increasing disease stage was associated with an exponential decrease in 123I-ioflupane uptake (R 2 = 0.1501) particularly in the head of the ipsilateral caudate nucleus (p < 0.0001), whereas increasing disease duration was associated with a linear decrease in 123I-ioflupane uptake (p < 0.0001; R 2 = 0.1532) particularly in the contralateral anterior putamen (p < 0.0001).
Conclusion
We observed two distinct spatiotemporal patterns of posterior to anterior dopaminergic depletion associated with disease stage and disease duration in patients with PD. The developed operator-independent reference database of 411 123I-ioflupane SPECT scans can be used for clinical and research applications.


Similar content being viewed by others
References
Italian Neurological Society, Italian Society of Clinical Neurophysiology. Guidelines for the Treatment of Parkinson’s Disease. The diagnosis of Parkinson’s disease. Neurol Sci. 2003;24(3 Suppl):S157–64.
Scherfler C, Seppi K, Donnemiller E, Goebel G, Brenneis C, Virgolini I, et al. Voxel-wise analysis of [123I]beta-CIT SPECT differentiates the Parkinson variant of multiple system atrophy from idiopathic Parkinson’s disease. Brain. 2005;128:1605–12.
Brücke PD, Asenbaum S, Pirker W, Djamshidian S, Wenger S, Wöber C, et al. Measurement of the dopaminergic degeneration in Parkinson’s disease with [123I]β-CIT and SPECT. J Neural Transm Suppl. 1997;50:9–24.
Sixel-Döring F, Liepe K, Mollenhauer B, Trautmann E, Trenkwalder C. The role of 123I-FP-CIT-SPECT in the differential diagnosis of Parkinson and tremor syndromes: a critical assessment of 125 cases. J Neurol. 2011;258:2147–54.
Kägi G, Bhatia KP, Tolosa E. The role of DAT-SPECT in movement disorders. J Neurol Neurosurg Psychiatr. 2010;81:5–12.
Segovia F, Górriz JM, Ramirez J, Alvarez I, Jimenez-Hoyuela JM, Ortega SJ. Improved parkinsonism diagnosis using a partial least squares based approach. Med Phys. 2012;39:4395–403.
Tissingh G, Bergmans P, Booij J, Winogrodzka A, van Royen EA, Stoof JC, et al. Drug-naive patients with Parkinson’s disease in Hoehn and Yahr stages I and II show a bilateral decrease in striatal dopamine transporters as revealed by [123I]β-CIT SPECT. J Neurol. 1997;245:14–20.
Laruelle M, Wallace E, Seibyl JP, Baldwin RM, Zea Ponce Y, Zoghbi SS, et al. Graphical, kinetic, and equilibrium analyses of in vivo [123I] beta-CIT binding to dopamine transporters in healthy human subjects. J Cereb Blood Flow Metab. 1994;14:982–94.
Benamer HT, Patterson J, Wyper DJ, Hadley DM, Macphee GJ, Grosset DG. Correlation of Parkinson’s disease severity and duration with 123I-FP-CIT SPECT striatal uptake. Mov Disord. 2000;15:692–8.
Marek K, Innis R, van Dyck C, Fussell B, Early M, Eberly S, et al. [123I]β-CIT SPECT imaging assessment of the rate of Parkinson’s disease progression. Neurology. 2001;57:2089–94.
Djaldetti R, Treves TA, Ziv I, Melamed E, Lampl Y, Lorberboym M. Use of a single [123I]-FP-CIT SPECT to predict the severity of clinical symptoms of Parkinson disease. Neurol Sci. 2009;30:301–5.
Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–42.
Garibotto V, Montandon ML, Viaud CT, Allaoua M, Assal F, Burkhard PR, et al. Regions of interest-based discriminant analysis of DaTSCAN SPECT and FDG-PET for the classification of dementia. Clin Nucl Med. 2013;38:e112–7.
Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–6.
Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–56.
Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41.
García-Gómez FJ, García-Solís D, Luis-Simón FJ, Marín-Oyaga VA, Carrillo F, Mir P, et al. Elaboration of the SPM template for the standardization of SPECT images with 123I-Ioflupane. Rev Esp Med Nucl Imagen Mol (Engl Ed). 2013;32:350–6.
Kas A, Payoux P, Habert M-O, Malek Z, Cointepas Y, El Fakhri G, et al. Validation of a standardized normalization template for statistical parametric mapping analysis of 123I-FP-CIT images. J Nucl Med. 2007;48:1459–67.
Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98.
Morrish PK, Sawle GV, Brooks DJ. An [F-18]dopa-PET and clinical study of the rate of progression in Parkinson’s disease. Brain. 1996;119:585–91.
Seibyl JP, Marchek KL, Quinlan D, Sheff K, Zoghbi S, Zea Ponce Y, et al. Decreased single‐photon emission computed tomographic {123I}β‐CIT striatal uptake correlates with symptom severity in Parkinson’s disease. Ann Neurol. 1995;38:589–98.
Ishikawa T, Dhawan V, Kazumata K, Chaly T, Mandel F, Neumeyer J, et al. Comparative nigrostriatal dopaminergic imaging with iodine-123-beta CIT-FP/SPECT and fluorine-18-FDOPA/PET. J Nucl Med. 1996;37:1760–5.
Booij J, Tissingh G, Winogrodzka A, Boer GJ, Stoof JC, Wolters EC, et al. Practical benefit of [123I]FP-CIT SPET in the demonstration of the dopaminergic deficit in Parkinson’s disease. Eur J Nucl Med. 1997;24:68–71.
Tissingh G, Booij J, Bergmans P, Winogrodzka A, Janssen A, van Royen EA, et al. Iodine-123-N-omega-fluoropropyl-2 beta-carbomethoxy-3 beta-(4-iodophenyl)tropane SPECT in healthy controls and early-stage, drug-naive Parkinson’s disease. J Nucl Med. 1998;39:1143–8.
Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications. N Engl J Med. 1988;318:876–80.
Seibyl JP, Marek KL, Quinlan D, Sheff K, Zoghbi S. Decreased SPECT [123I]beta-CIT striatal uptake correlates with symptom severity in idiopathic Parkinson’s disease. Ann Neurol. 1995;38:589–98.
Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, et al. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain. 2013;136:2419–31.
Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–301.
van Dyck CH, Seibyl JP, Malison RT, Laruelle M, Zoghbi SS, Baldwin RM, et al. Age-related decline in dopamine transporters: analysis of striatal subregions, nonlinear effects, and hemispheric asymmetries. Am J Geriatr Psychiatry. 2002;10:36–43.
Lavalaye J, Booij J, Reneman L, Habraken JB, van Royen EA. Effect of age and gender on dopamine transporter imaging with [123I]FP-CIT SPET in healthy volunteers. Eur J Nucl Med. 2000;27:867–9.
Martinez-Martin P, Gil-Nagel A, Gracia LM, Gómez JB, Martínez-Sarriés J, Bermejo F. Unified Parkinson’s disease rating scale characteristics and structure. The Cooperative Multicentric Group. Mov Disord. 1994;9:76–83.
Laruelle M, Baldwin RM, Malison RT, Zea-Ponce Y, Zoghbi SS, al-Tikriti MS, et al. SPECT imaging of dopamine and serotonin transporters with [123I]β-CIT: pharmacological characterization of brain uptake in nonhuman primates. Synapse. 1993;13:295–309.
Innis RB, Marek KL, Sheff K, Zoghbi S, Castronuovo J, Feigin A, et al. Effect of treatment with L-dopa/carbidopa or L-selegiline on striatal dopamine transporter SPECT imaging with [123I]β-CIT. Mov Disord. 1999;14:436–42.
Ahlskog JE, Uitti RJ, O’Connor MK, Maraganore DM, Matsumoto JY, Stark KF, et al. The effect of dopamine agonist therapy on dopamine transporter imaging in Parkinson’s disease. Mov Disord. 1999;14:940–6.
Varrone A, Dickson JC, Tossici-Bolt L, Sera T, Asenbaum S, Booij J, et al. European multicentre database of healthy controls for [123I]FP-CIT SPECT (ENC-DAT): age-related effects, gender differences and evaluation of different methods of analysis. Eur J Nucl Med Mol Imaging. 2012;40:213–27.
Nobili F, Naseri M, De Carli F, Asenbaum S, Booij J, Darcourt J, et al. Automatic semi-quantification of [123I]FP-CIT SPECT scans in healthy volunteers using BasGan version 2: results from the ENC-DAT database. Eur J Nucl Med Mol Imaging. 2012;40:565–73.
Compliance with ethical standards
ᅟ
Conflicts of interest
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was waived by the local ethics committee (retrospective analysis of imaging data acquired during clinical work-up).
Author information
Authors and Affiliations
Corresponding author
Additional information
Keypoints
• Disease stage and disease duration have different spatiotemporal patterns of dopaminergic depletion in PD.
• 123I-Ioflupane uptake in the striatum decreases exponentially with disease stages.
• Disease stage correlates particularly with uptake in the head of the caudate nucleus with ipsilateral predominance.
• 123I-ioflupane uptake in the striatum decreases linearly with disease duration.
• Disease duration correlates particularly with uptake in the anterior putamen with contralateral predominance.
• The operator-independent spatial normalization of 123I-ioflupane SPECT scans provides a reference database for research and clinical studies based on a large sample of 411 patients.
Rights and permissions
About this article
Cite this article
Badoud, S., Nicastro, N., Garibotto, V. et al. Distinct spatiotemporal patterns for disease duration and stage in Parkinson’s disease. Eur J Nucl Med Mol Imaging 43, 509–516 (2016). https://doi.org/10.1007/s00259-015-3176-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-015-3176-5