Abstract
Purpose
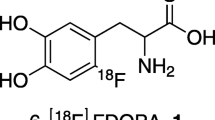
6-[18F]Fluorodopamine (4-(2-aminoethyl)-5-[18F]fluorobenzene-1,2-diol, 6-[18F]FDA) is a tracer for imaging sympathetically innervated tissues. Previous electrophilic labelling methods produced 6-[18F]FDA with low specific radioactivity (SA) which has limited its wider use. Our aim was to employ electrophilic labelling and increase the SA to around 15 GBq/μmol. We also sought to determine an extensive biodistribution pattern for 6-[18F]FDA in rats in order to thoroughly identify tissues with dense sympathetic innervation that were specifically labelled with 6-[18F]FDA. In addition, to investigate the safety profile of 6-[18F]FDA in larger animals, we performed in vivo studies in pigs.
Methods
6-[18F]FDA was synthesised using high SA electrophilic [18F]F2 as the labelling reagent. Biodistribution and metabolism of 6-[18F]FDA was determined ex vivo in rats, and in vivo studies were done in pigs.
Results
6-[18F]FDA was synthesised with 2.6 ± 1.1% radiochemical yield. The total amount of purified 6-[18F]FDA was 663 ± 291 MBq at the end of synthesis (EOS). SA, decay corrected to EOS, was 13.2 ± 2.7 GBq/μmol. Radiochemical purity exceeded 99.0%. Specific uptake of 6-[18F]FDA was demonstrated in heart, lung, pancreas, adrenal gland, lower large intestine (LLI), eye, thyroid gland, spleen and stomach tissue. 6-[18F]FDA in rat plasma declined rapidly, with a half-life of 2 min, indicating fast metabolism. In vivo PET studies in pigs confirmed the tracer could be used safely without pharmacological effects.
Conclusion
6-[18F]FDA was synthesised with good radiopharmaceutical quality and yields high enough for several human PET studies. The SA of 6-[18F]FDA was improved by 50- to 500-fold compared to previous electrophilic methods. Uptake of 6-[18F]FDA was specific in various peripheral organs, indicating that 6-[18F]FDA PET can be used to investigate sympathoneural functions beyond cardiac studies when higher specific uptake is achieved.






Similar content being viewed by others
References
Raffel DM, Wieland DM. Assessment of cardiac sympathetic nerve integrity with positron emission tomography. Nucl Med Biol 2001;28:541–59.
Langer O, Halldin C. PET and SPET tracers for mapping the cardiac nervous system. Eur J Nucl Med Mol Imaging 2002;29:416–34.
Eisenhofer G, Hovevey-Sion D, Kopin IJ, Miletich R, Kirk KL, Finn R, et al. Neuronal uptake and metabolism of 2- and 6-fluorodopamine: false neurotransmitters for positron emission tomographic imaging of sympathetically innervated tissues. J Pharmacol Exp Ther 1989;248:419–27.
Kirk KL. Photochemistry of diazonium salts. Synthesis of ring-fluorinated tyramines and dopamines. J Org Chem 1976;41:2373–6.
Goldberg LI, Kohli JD, Cantacuzene D, Kirk KI, Creveling CR. Effects of ring fluorination on the cardiovascular actions of dopamine and norepinephrine in the dog. J Pharmacol Exp Ther 1980;213:509–13.
Goldstein DS, Chang PC, Eisenhofer G, Miletich R, Finn R, Bacher J, et al. Positron emission tomographic imaging of cardiac sympathetic innervation and function. Circulation 1990;81:1606–21.
Rufini V, Calcagni ML, Baum RP. Imaging of neuroendocrine tumors. Semin Nucl Med 2006;36:228–47.
Ilias I, Yu J, Carrasquillo JA, Chen CC, Eisenhofer G, Whatley M, et al. Superiority of 6-[18F]-fluorodopamine positron emission tomography versus [131I]-metaiodobenzylguanidine scintigraphy in the localization of metastatic pheochromocytoma. J Clin Endocrinol Metab 2003;88:4083–7.
Ilias I, Chen CC, Carrasquillo JA, Whatley M, Ling A, Lazúrova I, et al. Comparison of 6-18F-fluorodopamine PET with 123I-metaiodobenzylguanidine and 111In-pentetreotide scintigraphy in localization of nonmetastatic and metastatic pheochromocytoma. J Nucl Med 2008;49:1613–9.
Goldstein DS, Grossman E, Tamrat M, Chang PC, Eisenhofer G, Bacher J, et al. Positron emission imaging of cardiac sympathetic innervation and function using 18F-6-fluorodopamine: effects of chemical sympathectomy by 6-hydroxydopamine. J Hypertens 1991;9:417–23.
Goldstein DS, Eisenhofer G, Dunn BB, Armando I, Lenders J, Grossman E, et al. Positron emission tomographic imaging of cardiac sympathetic innervation using 6-[18F]fluorodopamine: initial findings in humans. J Am Coll Cardiol 1993;22:1961–71.
Goldstein DS, Holmes C, Cannon RO, Eisenhofer G, Kopin IJ. Sympathetic cardiomyopathy in dysautonomias. N Engl J Med 1997;336:696–702.
Goldstein DS, Holmes C, Stuhlmuller JE, Lenders JWM, Kopin IJ. 6-[18F]fluorodopamine positron emission tomography scanning in the assessment of cardiac sympathoneural function—studies in normal humans. Clin Auton Res 1997;7:17–29.
Chiueh CC, Zukowska-Grojec Z, Kirk KL, Kopin IJ. 6-Fluorocathecolamines as false adrenergic neurotransmitters. J Pharmacol Exp Ther 1983;225:529–33.
Ding Y-S, Fowler JS, Gatley SJ, Dewey SL, Wolf AP, Schlyer DJ. Synthesis of high specific activity 6-[18F]fluorodopamine for positron emission tomography studies of sympathetic nervous tissue. J Med Chem 1991;34:861–3.
Chirakal R, Coates G, Firnau G, Schrobilgen GJ, Nahmias C. Direct radiofluorination of dopamine: 18F-labeled 6-fluorodopamine for imaging cardiac sympathetic innervation in humans using positron emission tomography. Nucl Med Biol 1996;23:41–5.
Chaly T, Dahl R, Matacchieri R, Bandyopadhyay D, Belakhlef A, Dhawan V, et al. Synthesis of 6-[18F]fluorodopamine with a synthetic unit made up of primarily sterile disposable components and operated by a master slave manipulator. Appl Radiat Isot 1993;44:869–73.
Namavari M, Satyamurthy N, Barrio JR. Synthesis of 6-[18F]fluorodopamine, 6-[18F]fluoro-m-tyramine and 4-[18F]fluoro-m-tyramine. J Labelled Comp Radiopharm 1995;36:825–33.
Dunn B, Channing MA, Adams HR, Goldstein DS, Kirk KL, Kiesewetter DO. A single column, rapid quality control procedure for 6-[18F]-fluoro-L-dopa and 6-[18F]fluorodopamine PET imaging agents. Int J Rad Appl Instrum B 1991;18:209–13.
Bergman J, Solin O. Fluorine-18-labeled fluorine gas for synthesis of tracer molecules. Nucl Med Biol 1997;24:677–83.
Lasne M-C, Perrio C, Rouden J, Barré L, Roeda D, Dolle F, et al. Chemistry of β+-emitting compounds based on fluorine-18. Top Curr Chem 2002;222:201–58.
Goldstein DS, Coronado L, Kopin IJ. 6-[Fluorine-18]fluorodopamine pharmacokinetics and dosimetry in humans. J Nucl Med 1994;35:964–73.
Chang P, Szemeredi K, Grossman E, Kopin I, Goldstein DS. Fate of tritiated 6-fluorodopamine in rats: a false neurotransmitter for positron emission tomographic imaging of sympathetic innervation and function. J Pharmacol Exp Ther 1990;255:809–17.
Pacak K, Eisenhofer G, Goldstein DS. Functional imaging of endocrine tumors: role of positron emission tomography. Endocr Rev 2004;25:568–80.
Goldstein DS, Chang PC, Smith CB, Herscovitch P, Austin SM, Eisenhofer G, et al. Dosimetric estimates for clinical positron emission tomographic scanning after injection of [18F]-6-fluorodopamine. J Nucl Med 1991;32:102–10.
Ding Y-S, Fowler JS, Dewey SL, Logan J, Schlyer DJ, Gatley J, et al. Comparison of high specific activity (-) and (+)-6-[18F]fluoronorepinephrine and 6-[18F]fluorodopamine in baboons: heart uptake, metabolism and the effect of desipramine. J Nucl Med 1993;34:619–29.
Brunello N, Mendlewicz J, Kasper S, Leonard B, Montgomery S, Craig Nelson J, et al. The role of noradrenaline and selective noradrenaline reuptake inhibition in depression. Eur Neuropsychopharmacol 2002;12:461–75.
Bellinger DL, Millar BA, Perez S, Carter J, Wood C, ThyagaRajan S, et al. Sympathetic modulation of immunity: relevance to disease. Cell Immunol 2008;252:27–56.
Magee DF. Does the sympathetic nervous system regulate the exocrine pancreas? Int J Pancreatol 1989;5:109–16.
Sundler F, Grunditz T, Håkanson R, Uddman R. Innervation of the thyroid. A study of the rat using retrograde tracing and immunocytochemistry. Acta Histochem Suppl 1989;37:191–8.
Tsunoda M. Recent advances in methods for the analysis of catecholamines and their metabolites. Anal Bioanal Chem 2006;386:506–14.
Goldstein DS, Holmes C. Metabolic fate of the sympathoneural imaging agent 6-[18F]fluorodopamine in humans. Clin Exp Hypertens 1997;19:155–61.
Wang RF, Loc’h C, Mazière B. Determination of unchanged [18F]dopamine in human and non-human primate plasma during positron emission tomography studies: a new solid-phase extraction method comparable to radio-thin-layer chromatography analysis. J Chromatogr B Biomed Sci Appl 1997;693:265–70.
Goldstein DS, Katzper M, Linares O, Kopin IJ. Kinetic model for the fate of 6-[18F]fluorodopamine in the human heart: a novel means to examine cardiac sympathetic neuronal function. Naunyn Schmiedebergs Arch Pharmacol 2002;365:38–49.
Acknowledgements
We thank Professor Kenneth Kirk for kindly supplying us with 2-fluorodopamine and 6-fluorodopamine free of charge for reference purposes.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eskola, O., Grönroos, T.J., Naum, A. et al. Novel electrophilic synthesis of 6-[18F]fluorodopamine and comprehensive biological evaluation. Eur J Nucl Med Mol Imaging 39, 800–810 (2012). https://doi.org/10.1007/s00259-011-2032-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-011-2032-5