Abstract
Purpose
Bone-seeking radiopharmaceuticals have palliative benefit in castration-resistant prostate cancer (CRPC) metastatic to bone. Recent studies have shown improvement of survival and quality of life when radiopharmaceuticals were given repeatedly or in combination with chemotherapy. We designed a phase I study combining docetaxel and 186Re-labelled hydroxyethylidene diphosphonate (HEDP) in men with CRPC and bone metastases to evaluate toxicity.
Methods
A dose escalation schedule was designed consisting of four dose levels with a standard dosage of docetaxel (75 mg/m2 3-weekly). 186Re-HEDP was given in increasing activities (1,250 MBq up to 2,500 MBq) after the third and sixth cycle of docetaxel. Dose limiting toxicity (DLT) was defined as any grade 4 toxicity lasting more than 7 days or any grade 3 toxicity that did not recover within 10 days. Three patients were planned for each dose level expanding to six if a DLT occurred.
Results
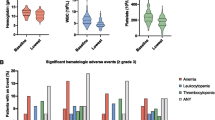
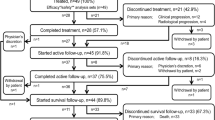
Fourteen patients were recruited with a median age of 64.6 years. One DLT, grade 3 thrombocytopenia lasting >10 days, occurred at dose level 3 leading to expansion of this group to six. One of these patients had an episode of acute renal failure which resolved. Because of production problems of 186Re-HEDP dose level 4 was not started.
Conclusion
Combined therapy with docetaxel and 186Re-HEDP is generally well tolerated in patients with CRPC metastatic to bone. We will conduct a randomized phase II study using three cycles of docetaxel 75 mg/m2 3-weekly followed by 188Re-HEDP 40 MBq/kg body weight, followed by another three cycles of docetaxel 75 mg/m2, followed by 188Re-HEDP 20 MBq/kg body weight.
Similar content being viewed by others
References
Vogelzang NJ, Crawford ED, Zietman A. Current clinical trial design issues in hormone-refractory prostate carcinoma. Consensus Panel. Cancer 1998;82:2093–101.
Clarke NW. Management of the spectrum of hormone refractory prostate cancer. Eur Urol 2006;50:428–39.
Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004;351:1502–12.
Petrylak DP, Tangen CM, Hussain MH, Lara Jr PN, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004;351:1513–20.
Roqué M, Martinez MJ, Alonso P, Català E, Garcia JL, Ferrandiz M. Radioisotopes for metastatic bone pain. Cochrane Database Syst Rev 2003;4:CD003347.
Han SH, de Klerk JM, Tan S, van het Schip AD, Derksen BH, van Dijk A, et al. The PLACORHEN study: a double-blind, placebo-controlled, randomized radionuclide study with (186)Re-etidronate in hormone-resistant prostate cancer patients with painful bone metastases. Placebo Controlled Rhenium study. J Nucl Med 2002;43:1150–6.
Palmedo H, Manka-Waluch A, Albers P, Schmidt-Wolf IG, Reinhardt M, Ezziddin S, et al. Repeated bone-targeted therapy for hormone-refractory prostate carcinoma: randomized phase II trial with the new, high-energy radiopharmaceutical rhenium-188 hydroxyethylidenediphosphonate. J Clin Oncol 2003;21:2869–75.
O’Sullivan JM, Norman AR, McCready VR, Flux G, Buffa FM, Johnson B, et al. A phase 2 study of high-activity 186Re-HEDP with autologous peripheral blood stem cell transplant in progressive hormone-refractory prostate cancer metastatic to bone. Eur J Nucl Med Mol Imaging 2006;33:1055–61.
Tu SM, Millikan RE, Mengistu B, Delpassand ES, Amato RJ, Pagliaro LC, et al. Bone-targeted therapy for advanced androgen-independent carcinoma of the prostate: a randomised phase II trial. Lancet 2001;357:336–41.
Morris MJ, Pandit-Taskar N, Carrasquillo J, Divgi CR, Slovin S, Kelly WK, et al. Phase I study of samarium-153 lexidronam with docetaxel in castration-resistant metastatic prostate cancer. J Clin Oncol 2009;27:2436–42.
Fizazi K, Beuzeboc P, Lumbroso J, Haddad V, Massard C, Gross-Goupil M, et al. Phase II trial of consolidation docetaxel and samarium-153 in patients with bone metastases from castration-resistant prostate cancer. J Clin Oncol 2009;27:2429–35.
Lam MG, Bosma TB, van Rijk PP, Zonnenberg BA. (188)Re-HEDP combined with capecitabine in hormone-refractory prostate cancer patients with bone metastases: a phase I safety and toxicity study. Eur J Nucl Med Mol Imaging 2009;36:1425–33.
Tu SM, Mathew P, Wong FC, Jones D, Johnson MM, Logothetis CJ. Phase I study of concurrent weekly docetaxel and repeated samarium-153 lexidronam in patients with castration-resistant metastatic prostate cancer. J Clin Oncol 2009;27:3319–24.
Lam MG, de Klerk JM, van Rijk PP, Zonnenberg BA. Bone seeking radiopharmaceuticals for palliation in cancer patients with osseous metastases. Anticancer Agents Med Chem 2007;7:381–97.
Lam MG, Hoekstra A, de Klerk JM, van Rijk PP, Zonnenberg BA. Radiation safety considerations for the bone seeking radiopharmaceuticals 89SrCl2, 186Re-HEDP and 153Sm-EDTMP. Nuklearmedizin 2009;48:37–43.
Han SH, de Klerk JM, Zonnenberg BA, Tan S, van Rijk PP. 186Re-etidronate. Efficacy of palliative radionuclide therapy for painful bone metastases. Q J Nucl Med 2001;45:84–90.
de Klerk JM, Zonnenberg BA, van het Schip AD, van Dijk A, Huiskes AW, van Rijk PP. Can bone marrow scintigraphy predict platelet toxicity after treatment with 186Re-HEDP? Nucl Med Commun 1999;20:833–6.
Han SH, Zonnenberg BA, de Klerk JM, Quirijnen JM, van het Schip AD, van Dijk A, et al. 186Re-etidronate in breast cancer patients with metastatic bone pain. J Nucl Med 1999;40:639–42.
De Klerk JM, Zonnenberg BA, Blijham GH, van Het Schip AD, Hoekstra A, Han SH, et al. Treatment of metastatic bone pain using the bone seeking radiopharmaceutical Re-186-HEDP. Anticancer Res 1997;17:1773–7.
Quirijnen JM, Han SH, Zonnenberg BA, de Klerk JM, van het Schip AD, van Dijk A, et al. Efficacy of rhenium-186-etidronate in prostate cancer patients with metastatic bone pain. J Nucl Med 1996;37:1511–5.
de Klerk JM, van het Schip AD, Zonnenberg BA, van Dijk A, Quirijnen JM, Blijham GH, et al. Phase 1 study of rhenium-186-HEDP in patients with bone metastases originating from breast cancer. J Nucl Med 1996;37:244–9.
de Klerk JM, van Dieren EB, van het Schip AD, Hoekstra A, Zonnenberg BA, van Dijk A, et al. Bone marrow absorbed dose of rhenium-186-HEDP and the relationship with decreased platelet counts. J Nucl Med 1996;37:38–41.
de Klerk JM, Zonnenberg BA, van het Schip AD, van Dijk A, Han SH, Quirijnen JM, et al. Dose escalation study of rhenium-186-hydroxyethylidene diphosphonate in patients with metastatic prostate cancer. Eur J Nucl Med 1994;21:1114–20.
de Klerk JM, van het Schip AD, Zonnenberg BA, van Dijk A, Stokkel MP, Han SH, et al. Evaluation of thrombocytopenia in patients treated with rhenium-186-HEDP: guidelines for individual dosage recommendations. J Nucl Med 1994;35:1423–8.
de Klerk JM, van Dijk A, van het Schip AD, Zonnenberg BA, van Rijk PP. Pharmacokinetics of rhenium-186 after administration of rhenium-186-HEDP to patients with bone metastases. J Nucl Med 1992;33:646–51.
Buffa FM, Flux GD, Guy MJ, O’Sullivan JM, McCready VR, Chittenden SJ, et al. A model-based method for the prediction of whole-body absorbed dose and bone marrow toxicity for 186Re-HEDP treatment of skeletal metastases from prostate cancer. Eur J Nucl Med Mol Imaging 2003;30:1114–24.
O’Sullivan JM, McCready VR, Flux G, Norman AR, Buffa FM, Chittenden S, et al. High activity rhenium-186-HEDP with autologous peripheral blood stem cell rescue: a phase I study in progressive hormone refractory prostate cancer metastatic to bone. Br J Cancer 2002;86:1715–20.
Liepe K, Kotzerke J. A comparative study of 188Re-HEDP, 186Re-HEDP, 153Sm-EDTMP and 89Sr in treatment of painful skeletal metastases. Nucl Med Commun 2007;28:623–30.
Palmedo H, Guhlke S, Bender H, Sartor J, Schoeneich G, Risse J, et al. Dose escalation study with rhenium-188 hydroxyethylidene diphosphonate in prostate cancer patients with osseous metastases. Eur J Nucl Med 2000;27:123–30.
Acknowledgements
This research was supported by unrestricted grants from Sanofi Aventis and Tyco Mallinckrodt. The staff working on this project in Belfast were supported through the UK Experimental Cancer Medicine Centre initiative. The Belfast Experimental Cancer Medicine Centre is supported by a programme grant from Cancer Research UK and the N. Ireland Health and Social Care Research and Development Division. We are grateful to the staff in both our sites for their help. Above all, we thank our patients, their families and friends for their support and participation in this clinical trial.
Conflicts of interest
Joe M. O’Sullivan and Richard H. Wilson received honoraria from Sanofi Aventis. In addition as the principal investigator Joe M. O'Sullivan received research funding from Sanofi Aventis and Mallinckrodt/Covidien. The other authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van Dodewaard-de Jong, J.M., de Klerk, J.M.H., Bloemendal, H.J. et al. A phase I study of combined docetaxel and repeated high activity 186Re-HEDP in castration-resistant prostate cancer (CRPC) metastatic to bone (the TAXIUM trial). Eur J Nucl Med Mol Imaging 38, 1990–1998 (2011). https://doi.org/10.1007/s00259-011-1883-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-011-1883-0