Abstract
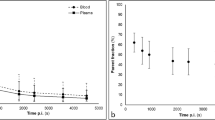
Fluorine-18 3′-deoxy-3′-fluorothymidine (18FLT) is a tissue proliferation marker which has been suggested as a new tumour-specific imaging tracer in positron emission tomography (PET). The objectives of this study were to investigate the pharmacokinetics of 18FLT in patients with colorectal cancer, defining methodologies for the quantitative analysis of the in vivo 18FLT uptake and subsequently assessing the accuracy of semi-quantitative measures. Dynamic acquisitions over a single field of view of interest identified by computed tomography were carried out for up to 60 min following injection of 18FLT (360±25 MBq). Dynamic arterial blood sampling was carried out in order to provide a blood input function. Simultaneous venous samples were also taken in order to investigate their potential utilisation in deriving a hybrid input function. Arterial and venous blood samples at 5, 15, 30, 60 and 90 min p.i. were used for metabolite analysis. Eleven patients with primary and/or metastatic colorectal cancer were studied on a lesion by lesion basis (n=21). All acquired images were reconstructed using ordered subsets expectation maximisation and segmented attenuation correction. Time-activity curves were derived by image region of interest (ROI) analysis and image-based input functions were obtained using abdominal or thoracic aorta ROIs. Standardised uptake values (SUVs) were calculated to provide semi-quantitative indices of uptake, while non-linear regression (NLR) methodology in association with a three-compartment model and Patlak analysis were carried out to derive the net influx constant K i . The metabolite analysis revealed two radioactive metabolites, with the parent compound representing ~80% of the total radioactivity in the 30-min plasma sample. In the case of NLR, better fits were obtained with a 3k model (i.e. k 4=0) for both lesion and bone marrow time-activity curves. For the same lesions, a high correlation was observed between the K i derived from either Patlak analysis or NLR(3k) and the corresponding SUVs. Our results also suggest that the quantitative behaviour of 18FLT in vivo (up to 60 min p.i.) may be characterised using a 3k model or Patlak analysis in combination with image-derived input functions. The good correlation found between the SUVs (at 60 min) and K i values supports the use of semi-quantitative indices to assess the proliferation rate of colorectal cancer lesions in vivo with 18FLT.









Similar content being viewed by others
References
Bomanji JB, Costa DC, Ell PJ. Clinical role of positron emission tomography in oncology. Lancet Oncol 2001; 2:157–164.
Gambhir SS, Czernin J, Schwimmer J, Silverman DHS, Coleman RE, Phelps ME. A tabulated summary of the FDG PET literature. J Nucl Med 2001; 42:1S–93S.
Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics, 1997. CA Cancer J Clin 1997; 47:5–27.
Galandiuk S, Wieland HS, Moertel CG, et al. Patterns of recurrence after curative resection of carcinoma of the colon and rectum. Surg Gynecol Obstet 1992; 174:27–32.
Thoeni RF. Colorectal cancer. Radiologic staging. Radiol Clin North Am 1997; 35:457–485.
Ruers TJM, Langenhoff BS, Neeleman GJ, Jager GJ, Strijk S, Wobbes T, Corstens FHM, Oyen WJG. Value of positron emission tomography with [18F]fluorodeoxyglucose in patients with colorectal liver metastases: a prospective study. J Clin Oncol 2002; 20:388–395.
Zeally IA, Skehan SJ, Rawlinson J, Coates G, Nahmias C, Somers S. Selection of patients for resection of hepatic metastases: improved detection of extra-hepatic disease with FDG PET. Radiographics 2001; 21:S55–S69.
Arulampalam T, Costa DC, Visvikis D, Boulos P, Taylor I, Ell PJ. The impact of FDG-PET on the management algorithm for recurrent colorectal cancer. Eur J Nucl Med 2001; 28:1758–1765.
Swanson RS. Is an FDG-PET scan the new imaging standard for colon cancer? Ann Surg Oncol 2001; 8:752–753.
Kubota R, Yamada S, Kubota K, Ishiwata K, Tamahashi N, Ido T. Intratumoral distribution of18F-fluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med 1992; 33:1972–1980.
Bakheet SM, Powe J, Kandil A. F-18 FDG uptake in breast infection and inflammation. Clin Nucl Med 2000; 25:100–103.
Shreve PD. Focal fluorine-18 fluorodeoxyglucose accumulation in inflammatory pancreatic disease. Eur J Nucl Med 1998; 25:259–264.
Shreve PD, Anzai Y, Wahl RL. Pitfalls in oncologic diagnosis with FDG PET imaging: physiologic and benign variants. Radiographics 1999; 19:61–77.
Shields AF, Mankoff DA, Link JM, Graham MW, Eary JF, Kozawa SM, Zheng M, Lewellen B, Lewellen TK, Grierson JR, Krohn KA. carbon-11-thymidine and FDG to measure therapy response. J Nucl Med 1998; 39:1757–1762.
Kalff V, Hicks RJ, Ware RE, Hogg A, Binns D, McKenzie AF. The clinical impact of18F-FDG PET in patients with suspected or confirmed recurrence of colorectal cancer: a prospective study. J Nucl Med 2002; 43:492–499.
Huebner RH, Park KC, Shepherd JE, Schwimmer J, Czernin J, Phelps ME, Gambhir SS. A meta-analysis of the literature for whole body FDG PET detection of recurrent colorectal cancer. J Nucl Med 2000; 41:1177–1189.
Sundoro-Wu BM, Schmall B, Conti PS, et al. Selective alkylation of pyrimidyl-dianons: synthesis and purification of11C labeled thymidine for tumor visualization using positron emission tomography. Appl Radiat Isot 1985; 35:705–708.
Vander Borght T, Labar D, Pauwels S, Lambotte L. Production of [2-11C]thymidine for quantification of cellular proliferation with PET. Appl Radiat Isot 1991; 42:103–104.
Grierson JR, Shields AF. Radiosynthesis of 3′-deoxy-3′-[18F]fluorothymidine: [18F]FLT for imaging of cellular proliferation in vivo. Nucl Med Biol 2000; 27:143–156.
Machulla HJ, Blocker A, Kuntzsch M, Piert M, Wie R, Grierson JR. Simplified labeling approach for synthesizing 3′-deoxy-3′-[18F]fluorothymidine ([18F]FLT). J Radioanal Nucl Chem 2000; 243:843–846.
Martin SJ, Eisenbarth JA, Wagner-Utermann U, Mier W, Haberkorn U, Eisenhut M. [18F]FLT: 18F labeling of 3-Boc-1-(2-deoxy-3-O-nosyl-5-O-trityl-β-d-lyxofuranosyl)thymine and other thymine derivatives. J Nucl Med 2000; 41:255P.
Hengstschlager M, Knofler M, Mullner EW, Ogris E, Wintersberger E, Wawra E. Different regulation of thymidine kinase during the cell cycle of normal versus DNA tumor virus-transformed cells. J Biol Chem 1994; 269:13836–13942.
Toyohara J, Waki A, Takamatsu S, Yonekura Y, Magata Y, Fujibayashi Y. Basis of FLT as a cell proliferation marker: comparative uptake studies with [3H]thymidine and [3H]arabinothymidine, and cell analysis in 22 asynchronously growing tumor cell lines. Nucl Med Biol 2002; 29:281–287.
Shields AF, Dohmen BM, Mangner TJ, Lawhorn-Crews JM, Machulla H, Muzik O, Bares R. Imaging of thoracic tumors with18FLT. J Nucl Med 2000; 41:74P.
Buck AK, Schirrmeister H, Hetzel M, von der Heide M, Halter G, Glatting G, Mattfeldt T, Liewald F, Reske SN, Neumaier B. 3-Deoxy-3-[18F]fluorothymidine-positron emission tomography for noninvasive assessment of proliferation in pulmonary nodules. Cancer Res 2002; 62:3331–3334.
Shields AF, Grierson JR, Muzik O, Stayanoff JC, Lawhorn JM, Obradovich JE, Mangner TJ. Kinetics of 3-deoxy-3-[18F]fluorothymidine uptake and retention in dogs. Mol Imag Biol 2002; 4:83–89.
Visvikis D, Cheze-LeRest C, Costa DC, Bomanji J, Gacinovic S, Ell PJ. Influence of OSEM and segmented attenuation correction in the calculation of standardised uptake values for18FDG PET. Eur J Nucl Med 2001; 28:1326–1335.
Bettinardi V, Pagani E, Gilardi MC, Landoni C, Riddell C, Rizzo G, Castiglioni I, Belluzzo D, Lucignani G, Schubert S, Fazio F. An automatic classification technique for attenuation correction in positron emission tomography. Eur J Nucl Med 1999; 26:447–458.
DeGrado T, Turkington T, Williams J, Stearns C, Hoffman J. Performance characteristics of a whole body PET scanner. J Nucl Med 1994; 35:1398–1406.
Cleij MC, Steel CJ, Brady F, Ell PJ, Pike VW, Luthra SK. An improved synthesis of 3-deoxy-3-[18F]fluorothymidine. J Lab Comp Radiopharm 2001; 44.
Lodge MA, Lucas JD, Marsden PK, Cronin BF, O’Doherty MJ, Smith MA. A PET study of18FDG uptake in soft tissue masses. Eur J Nucl Med 1999; 26:22–30.
Eary JF, Mankoff DA. Tumor metabolic rates in sarcoma using FDG PET. J Nucl Med 1998; 39:250–254.
Ohtake T, Kosaka N, Watanabe T, Yokohama I, Moritan T, Masuo M, Iizuka M, Kozeni K, Momose T, Oku S, Nishikawa J, Sasaki Y, Iio M. Noninvasive method to obtain input function for measuring tissue glucose utilization of thoracic and abdominal organs. J Nucl Med 1991; 32:1432–1438.
Germano G, Chen BC, Huang SC, Gambhir SS, Hoffman EJ, Phelps ME. Use of the abdominal aorta for arterial input function determination in hepatic and renal PET studies. J Nucl Med 1992; 33:613–620.
van der Weerdt AP, Klein LJ, Boellaard R, Visser CA, Visser FC, Lammertsma AA. Image-derived input functions for determination of MRGlu in cardiac18F-FDG PET scans. J Nucl Med 2001; 42:1622–1629.
Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple time uptake data. J Cereb Blood Flow Metab 1983; 3:1–7.
Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple time uptake data: generalizations. J Cereb Blood Flow Metab 1985; 5:584–590.
Hoekstra CJ, Paglianiti I, Hoekstra OS, Smit EF, Postmus PE, Teule GJJ, Lammertsma AA. Monitoring response to therapy in cancer using18F-2-fluoro-2-deoxy-d-glucose and positron emission tomography: an overview of different analytical methods. Eur J Nucl Med 2000; 27:731–743.
Phelps ME, Mazziotta JC, Schelbert HR. Positron emission tomography and autoradiography (principles and applications for the brain and heart). New York: Raven, 1986.
Akaike H. A new look at the statistical identification. IEEE Trans Autom Contr 1978; 19:716–723.
Schwarz G. Estimating the dimension of a model. Ann Stat 1978; 6:461–464.
Shields AF, Dohmen BM, Mangner TJ, Kuntzsch M, Bares R, Stayanoff J, Muzik O, Machulla HJ. Metabolism of18FLT in patients. J Nucl Med 2000; 41:36P.
Francis DF, Visvikis D, Costa DC, Arulampalam T, Townsend C, Luthra SK, Taylor I, Ell PJ. Potential impact of18F-FLT versus 18F-FDG in positron emission tomography for colorectal cancer. Eur J Nucl Med Mol Imaging 2003; 30:988–994.
Acknowledgements
The authors would like to thank Caroline Townsend, Ian Pigden, Stella Simms and Saiful Islam for the acquisition of some of the patient data presented in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
The work included in this paper was selected for consideration in the Marie-Curie award during the European Association of Nuclear Medicine 2002 meeting in Vienna.
Rights and permissions
About this article
Cite this article
Visvikis, D., Francis, D., Mulligan, R. et al. Comparison of methodologies for the in vivo assessment of 18FLT utilisation in colorectal cancer. Eur J Nucl Med Mol Imaging 31, 169–178 (2004). https://doi.org/10.1007/s00259-003-1339-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-003-1339-2