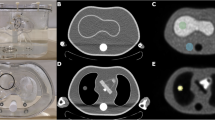
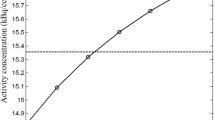
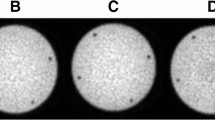
Abstract
The introduction of combined PET/CT systems has a number of advantages, including the utilisation of CT images for PET attenuation correction (AC). The potential advantage compared with existing methodology is less noisy transmission maps within shorter times of acquisition. The objective of our investigation was to assess the accuracy of CT attenuation correction (CTAC) and to study resulting bias and signal to noise ratio (SNR) in image-derived semi-quantitative uptake indices. A combined PET/CT system (GE Discovery LS) was used. Different size phantoms containing variable density components were used to assess the inherent accuracy of a bilinear transformation in the conversion of CT images to 511 keV attenuation maps. This was followed by a phantom study simulating tumour imaging conditions, with a tumour to background ratio of 5:1. An additional variable was the inclusion of contrast agent at different concentration levels. A CT scan was carried out followed by 5 min emission with 1-h and 3-min transmission frames. Clinical data were acquired in 50 patients, who had a CT scan under normal breathing conditions (CTACnb) or under breath-hold with inspiration (CTACinsp) or expiration (CTACexp), followed by a PET scan of 5 and 3 min per bed position for the emission and transmission scans respectively. Phantom and patient studies were reconstructed using segmented AC (SAC) and CTAC. In addition, measured AC (MAC) was performed for the phantom study using the 1-h transmission frame. Comparing the attenuation coefficients obtained using the CT- and the rod source-based attenuation maps, differences of 3% and <6% were recorded before and after segmentation of the measured transmission maps. Differences of up to 6% and 8% were found in the average count density (SUVavg) between the phantom images reconstructed with MAC and those reconstructed with CTAC and SAC respectively. In the case of CTAC, the difference increased up to 27% with the presence of contrast agent. The presence of metallic implants led to underestimation in the surrounding SUVavg and increasing non-uniformity in the proximity of the implant. The patient study revealed no statistically significant differences in the SUVavg between either CTACnb or CTACexp and SAC-reconstructed images. The larger differences were recorded in the lung. Both the phantom and the patient studies revealed an average increase of ~25% in the SNR for the CTAC-reconstructed emission images compared with the SAC-reconstructed images. In conclusion, CTACnb or CTACexp is a viable alternative to SAC for whole-body studies. With CTAC, careful consideration should be given to interpretation of images and use of SUVs in the presence of oral contrast and in the proximity of metallic implants.






Similar content being viewed by others
References
Bomanji JB, Costa DC, Ell PJ. Clinical role of positron emission tomography in oncology. Lancet Oncol 2001; 2:157–164.
Gambhir SS, Czernin J, Schwimmer J, Silverman DHS, Coleman RE, Phelps ME. A tabulated summary of the FDG PET literature. J Nucl Med 2001; 42:1S–93S.
Schiepers C, Penninckx F, De Vadder N, Merckx E, Mortelmans L, Bormans G, Marchal G, Filez L, Aerts R. Contribution of PET in the diagnosis of recurrent colorectal cancer: comparison with conventional imaging. Eur J Surg Oncol 1995; 21:517–522.
Pieterman RM, van Putten JWG, Meuzelaar JJ et al. Preoperative staging of non-small cell lung cancer with PET. N Engl J Med 2000; 343:254–261.
Weber WA, Ziegler SI, Thodtmann R, Hanauske AR, Schwaiger M. Reproducibility of metabolic measurements in malignant tumours using FDG PET. J Nucl Med 1999; 40:1771–1777.
Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, Pruim J, Price P. Measurement of clinical and subclinical tumour response using 18F-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. Eur J Cancer 1999; 35:1773–1782.
Xu EZ, Mullani NA, Gould LK, Anderson WL. A segmented attenuation correction for PET. J Nucl Med 1991; 32:161–165.
Meikle SR, Dahlbom M, Cherry SR. Attenuation correction using count-limited transmission data in positron emission tomography. J Nucl Med 1993; 34:143–150.
Xu M, Cutler PD, Luk WK. Adaptive segmented attenuation correction for whole body PET imaging. IEEE Trans Nucl Sci 1996; 43:331–336.
Bettinardi V, Pagani E, Gilardi MC, Landoni C, Riddell C, Rizzo G, Castiglioni I, Belluzzo D, Lucignani G, Schubert S, Fazio F. An automatic classification technique for attenuation correction in positron emission tomography. Eur J Nucl Med 1999; 26:447–458.
Visvikis D, Cheze-LeRest C, Costa DC, Bomanji J, Gacinovic S, Ell PJ. Influence of OSEM and segmented attenuation correction in the calculation of standardised uptake values for [18F]FDG PET. Eur J Nuc Med 2001; 28:1326–1335.
Guy MJ, Castellano-Smith IA, Flower MA, Flux GD, Ott RJ, Visvikis D. DETECT Dual Energy Transmission Estimation CT for improved attenuation correction in SPECT and PET. IEEE Trans Nucl Sci 1998; 45:1261–1267.
Kinahan PE, Townsend DW, Beyer T, Sashin D. Attenuation correction for a combined 3D PET/CT scanner. Med Phys 1998; 25:2046–2053.
Burger C, Goerres G, Schoenes S, Buck A, Lonn AHR, von Schulthess GK. PET attenuation coefficients from CT images: experimental evaluation of the transformation of CT into PET 511keV attenuation coefficients. Eur J Nucl Med 2002; 29:922–927.
Goerres GW, Kamel E, Heidelberg TNH, Schwitter MR, Burger C, von Schulthess. PET-CT image co-registration in the thorax: influence of respiration. Eur J Nucl Med 2002; 29:351–360.
Nakamoto Y, Osman M, Cohade C, Marshall LT, Links JM, Kohlmyer S, Wahl RL. PET/CT: comparison of quantitative tracer uptake between germanium and CT transmission attenuation-corrected images. J Nucl Med 2002; 43:1137–1143.
White DR, Darlinson RJ. Epoxy resin based tissue substitutes. Br J Radiol 1977; 50:599.
Jordan K. IEC emission phantom. Appendix. "Performance evaluation of positron emission tomographs". Medical and Public Health Research Programme of the European Community.
Phantomlab. RANDO® phantom. http://www.phantomlab.com/rando.html
Soret M, Feuardent J, Hapdey S, Buvat I. Impact of a mismatch between the spatial resolution of the emission and transmission scans on the standardised uptake values (SUV) measured in FDG PET. J Nucl Med 2002; 43:13P.
Wang G, Snyder DL, O'Sullivan JA, Vannier MW. Iterative deblurring for CT metal artifact reduction. IEEE Trans Med Imaging 1996; 15:657–664.
Acknowledgements
The authors would like to thank Caroline Townsend, Ian Pigden and Stella Simms for the acquisition of the patient data presented in this study. We would also like to thank Dr. Tim Fryer and the Wolfson Brain Imaging Centre in Cambridge for allowing the use of the IEC phantom during this study, as well as Daren Francis for the provision of the metal implants used in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Visvikis, D., Costa, D.C., Croasdale, I. et al. CT-based attenuation correction in the calculation of semi-quantitative indices of [18F]FDG uptake in PET. Eur J Nucl Med 30, 344–353 (2003). https://doi.org/10.1007/s00259-002-1070-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-002-1070-4