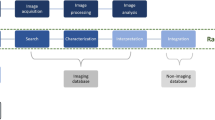
Abstract
Artificial intelligence (AI) is expected to bring greater efficiency in radiology by performing tasks that would otherwise require human intelligence, also at a much faster rate than human performance. In recent years, milestone deep learning models with unprecedented low error rates and high computational efficiency have shown remarkable performance for lesion detection, classification, and segmentation tasks. However, the growing field of AI has significant implications for radiology that are not limited to visual tasks. These are essential applications for optimizing imaging workflows and improving noninterpretive tasks. This article offers an overview of the recent literature on AI, focusing on the musculoskeletal imaging chain, including initial patient scheduling, optimized protocoling, magnetic resonance imaging reconstruction, image enhancement, medical image-to-image translation, and AI-aided image interpretation. The substantial developments of advanced algorithms, the emergence of massive quantities of medical data, and the interest of researchers and clinicians reveal the potential for the growing applications of AI to augment the day-to-day efficiency of musculoskeletal radiologists.




reproduced with permission from John Wiley and Sons. The figure is reprinted from Chaudhari et al. [59]

reproduced with permission from Elsevier. The figure is reprinted from Guirguis et al. [73]

reproduced with permission from Elsevier. The figure is reprinted from Cronin et al. [78]
Similar content being viewed by others
References
Langlotz CP, Allen B, Erickson BJ, Kalpathy-Cramer J, Bigelow K, Cook TS, et al. A roadmap for foundational research on artificial intelligence in medical imaging: from the 2018 NIH/RSNA/ACR/The Academy Workshop. Radiology. 2019;291(3):781–91.
Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25(1):44–56.
Chartrand G, Cheng PM, Vorontsov E, Drozdzal M, Turcotte S, Pal CJ, et al. Deep learning: a primer for radiologists. Radiographics. 2017;37(7):2113–31.
Lundervold AS, Lundervold A. An overview of deep learning in medical imaging focusing on MRI. Z Med Phys. 2019;29(2):102–27.
Qiu D, Zhang S, Liu Y, Zhu J, Zheng L. Super-resolution reconstruction of knee magnetic resonance imaging based on deep learning. Computer methods and programs in biomedicine. 2020;187:105059.
Wang X, Peng Y, Lu L, Lu Z, Bagheri M, Summers RM. Chestx-ray8: hospital-scale chest x-ray database and benchmarks on weakly-supervised classification and localization of common thorax diseases. In:Proceedings of the IEEE conference on computer vision and pattern recognition, 2017; 2097–106.
Knoll F, Zbontar J, Sriram A, Muckley MJ, Bruno M, Defazio A, et al. fastmri: a publicly available raw k-space and dicom dataset of knee images for accelerated mr image reconstruction using machine learning. Radiology: Artificial Intelligence. 2020;2(1):e190007.
Zbontar J, Knoll F, Sriram A, Muckley MJ, Bruno M, Defazio A, et al. fastMRI: an open dataset and benchmarks for accelerated MRI. arXiv preprint arXiv:1811.08839. 2018.
Rajpurkar P, Irvin J, Zhu K, Yang B, Mehta H, Duan T, et al. Chexnet: Radiologist-level pneumonia detection on chest x-rays with deep learning. arXiv preprint arXiv:1711.05225. 2017.
Esteva A, Kuprel B, Novoa RA, Ko J, Swetter SM, Blau HM, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542(7639):115–8.
Kermany DS, Goldbaum M, Cai W, Valentim CC, Liang H, Baxter SL, et al. Identifying medical diagnoses and treatable diseases by image-based deep learning. Cell. 2018;172(5):1122–31. e9.
LeCun Y, Bengio Y, Hinton G. Deep learning nature. 2015;521(7553):436–44.
Han Z, Wei B, Mercado A, Leung S, Li S. Spine-GAN: semantic segmentation of multiple spinal structures. Med Image Anal. 2018;50:23–35.
Chung SW, Han SS, Lee JW, Oh K-S, Kim NR, Yoon JP, et al. Automated detection and classification of the proximal humerus fracture by using deep learning algorithm. Acta Orthop. 2018;89(4):468–73.
Liu F, Zhou Z, Jang H, Samsonov A, Zhao G, Kijowski R. Deep convolutional neural network and 3D deformable approach for tissue segmentation in musculoskeletal magnetic resonance imaging. Magn Reson Med. 2018;79(4):2379–91.
Thomas KA, Kidziński Ł, Halilaj E, Fleming SL, Venkataraman GR, Oei EH, et al. Automated classification of radiographic knee osteoarthritis severity using deep neural networks. Radiology: Artificial Intelligence. 2020;2(2):e190065.
Weston AD, Korfiatis P, Kline TL, Philbrick KA, Kostandy P, Sakinis T, et al. Automated abdominal segmentation of CT scans for body composition analysis using deep learning. Radiology. 2019;290(3):669–79.
Richardson ML, Garwood ER, Lee Y, Li MD, Lo HS, Nagaraju A, et al. Noninterpretive uses of artificial intelligence in radiology. Academic Radiology. 2020.
Harvey HB, Liu C, Ai J, Jaworsky C, Guerrier CE, Flores E, et al. Predicting no-shows in radiology using regression modeling of data available in the electronic medical record. J Am Coll Radiol. 2017;14(10):1303–9.
Curran JS, Halpert RD, Straatman A. Patient, “no-shows”–a costly problem. Radiol Manage. 1989;11(1):20–3.
Goffman RM, Harris SL, May JH, Milicevic AS, Monte RJ, Myaskovsky L, et al. Modeling patient no-show history and predicting future outpatient appointment behavior in the veterans health administration. Mil Med. 2017;182(5–6):e1708–14.
Srinivas S, Ravindran AR. Optimizing outpatient appointment system using machine learning algorithms and scheduling rules: a prescriptive analytics framework. Expert Syst Appl. 2018;102:245–61.
Nelson A, Herron D, Rees G, Nachev P. Predicting scheduled hospital attendance with artificial intelligence. NPJ digital medicine. 2019;2(1):1–7.
Chong LR, Tsai KT, Lee LL, Foo SG, Chang PC. Artificial intelligence predictive analytics in the management of outpatient MRI appointment no-shows. Am J Roentgenol. 2020;215(5):1155–62.
Curtis C, Liu C, Bollerman TJ, Pianykh OS. Machine learning for predicting patient wait times and appointment delays. J Am Coll Radiol. 2018;15(9):1310–6.
Cheung YY, Goodman EM, Osunkoya TO. No more waits and delays: streamlining workflow to decrease patient time of stay for image-guided musculoskeletal procedures. Radiographics. 2016;36(3):856–71.
Zech J, Pain M, Titano J, Badgeley M, Schefflein J, Su A, et al. Natural language-based machine learning models for the annotation of clinical radiology reports. Radiology. 2018;287(2):570–80.
Wyles. Use of natural language processing algorithms to identify common data elements in operative notes for total hip arthroplasty (vol 101, pg 1931, 2019). Journal of Bone and Joint Surgery-American Volume. 2020;102(9).
Geis JR, Brady AP, Wu CC, Spencer J, Ranschaert E, Jaremko JL, et al. Ethics of artificial intelligence in radiology: summary of the Joint European and North American Multisociety Statement. Radiology. 2019;293(2):436–40.
Brown AD, Marotta TR. Using machine learning for sequence-level automated MRI protocol selection in neuroradiology. J Am Med Inform Assoc. 2018;25(5):568–71.
Kalra A, Chakraborty A, Fine B, Reicher J. Machine learning for automation of radiology protocols for quality and efficiency improvement. J Am Coll Radiol. 2020;17(9):1149–58.
Mantripragada VP, Muschler GF, Laprade RF. Variability in the preparation, reporting, and use of bone marrow aspirate concentrate in musculoskeletal disorders.
Lee YH. Efficiency improvement in a busy radiology practice: determination of musculoskeletal magnetic resonance imaging protocol using deep-learning convolutional neural networks. J Digit Imaging. 2018;31(5):604–10.
Trivedi H, Mesterhazy J, Laguna B, Vu T, Sohn JH. Automatic determination of the need for intravenous contrast in musculoskeletal MRI examinations using IBM Watson’s natural language processing algorithm. J Digit Imaging. 2018;31(2):245–51.
Richardson ML. MR protocol optimization with deep learning: a proof of concept. Current problems in diagnostic radiology. 2019.
Huang G, Liu Z, Van Der Maaten L, Weinberger KQ. Densely connected convolutional networks. In:Proceedings of the IEEE conference on computer vision and pattern recognition, 2017; 4700–8.
Kitamura G. Hanging protocol optimization of lumbar spine radiographs with machine learning. Skeletal Radiology. 2021.
Dratsch T, Korenkov M, Zopfs D, Brodehl S, Baessler B, Giese D, et al. Practical applications of deep learning: classifying the most common categories of plain radiographs in a PACS using a neural network. Eur Radiol. 2021;31(4):1812–8.
Karpathy A, Li FF. Deep visual-semantic alignments for generating image descriptions. IEEE Trans Pattern Anal Mach Intell. 2017;39(4):664–76.
Jing B, Xie P, Xing E. On the automatic generation of medical imaging reports. arXiv preprint arXiv:1711.08195. 2017.
van Ginneken B, Schaefer-Prokop CM, Prokop M. Computer-aided diagnosis: how to move from the laboratory to the clinic. Radiology. 2011;261(3):719–32.
Monshi MMA, Poon J, Chung V. Deep learning in generating radiology reports: a survey. Artificial Intelligence in Medicine. 2020;106.
Huang J, Shen H, Wu J, Hu X, Zhu Z, Lv X, et al. Spine Explorer: a deep learning based fully automated program for efficient and reliable quantifications of the vertebrae and discs on sagittal lumbar spine MR images. The Spine Journal. 2020;20(4):590–9.
McBee MP, Awan OA, Colucci AT, Ghobadi CW, Kadom N, Kansagra AP, et al. Deep learning in radiology. Acad Radiol. 2018;25(11):1472–80.
Li MD, Chang K, Bearce B, Chang CY, Huang AJ, Campbell JP, et al. Siamese neural networks for continuous disease severity evaluation and change detection in medical imaging. Npj Digital Medicine. 2020;3(1).
Dean DG. The role of MRI in musculoskeletal practice: a clinical perspective. Journal of Manual & Manipulative Therapy. 2011;19(3):152–61.
Lustig M, Donoho DL, Santos JM, Pauly JM. Compressed sensing MRI. IEEE Signal Process Mag. 2008;25(2):72–82.
Deshmane A, Gulani V, Griswold MA, Seiberlich N. Parallel MR imaging. J Magn Reson Imaging. 2012;36(1):55–72.
Lustig M, Donoho D, Pauly JM. Sparse MRI: the application of compressed sensing for rapid MR imaging. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2007;58(6):1182–95.
Wang S, Su Z, Ying L, Peng X, Zhu S, Liang F, et al. Accelerating magnetic resonance imaging via deep learning. In:2016 IEEE 13th International Symposium on Biomedical Imaging (ISBI): IEEE, 2016; 514–7.
Eo T, Jun Y, Kim T, Jang J, Lee HJ, Hwang D. KIKI-net: cross-domain convolutional neural networks for reconstructing undersampled magnetic resonance images. Magn Reson Med. 2018;80(5):2188–201.
Hammernik K, Klatzer T, Kobler E, Recht MP, Sodickson DK, Pock T, et al. Learning a variational network for reconstruction of accelerated MRI data. Magn Reson Med. 2018;79(6):3055–71.
Yang Y, Sun J, Li H, Xu Z. ADMM-CSNet: a deep learning approach for image compressive sensing. IEEE Trans Pattern Anal Mach Intell. 2018;42(3):521–38.
Recht MP, Zbontar J, Sodickson DK, Knoll F, Yakubova N, Sriram A, et al. Using deep learning to accelerate knee MRI at 3 T: results of an interchangeability study. Am J Roentgenol. 2020;215(6):1421–9.
Zhu B, Liu JZ, Cauley SF, Rosen BR, Rosen MS. Image reconstruction by domain-transform manifold learning. Nature. 2018;555(7697):487–92.
Tameem HZ, Sinha US. Automated image processing and analysis of cartilage MRI: enabling technology for data mining applied to osteoarthritis. In:AIP conference proceedings: American Institute of Physics, 2007; 262–76.
Hossain MB, Lai KW, Pingguan-Murphy B, Hum YC, Salim MIM, Liew YM. Contrast enhancement of ultrasound imaging of the knee joint cartilage for early detection of knee osteoarthritis. Biomed Signal Process Control. 2014;13:157–67.
Van Reeth E, Tham IW, Tan CH, Poh CL. Super-resolution in magnetic resonance imaging: a review. Concepts in Magnetic Resonance Part A. 2012;40(6):306–25.
Chaudhari AS, Fang Z, Kogan F, Wood J, Stevens KJ, Gibbons EK, et al. Super-resolution musculoskeletal MRI using deep learning. Magn Reson Med. 2018;80(5):2139–54.
Ledig C, Theis L, Huszár F, Caballero J, Cunningham A, Acosta A, et al. Photo-realistic single image super-resolution using a generative adversarial network. In:Proceedings of the IEEE conference on computer vision and pattern recognition, 2017; 4681–90.
Zhu J-Y, Park T, Isola P, Efros AA. Unpaired image-to-image translation using cycle-consistent adversarial networks. In:Proceedings of the IEEE international conference on computer vision, 2017; 2223–32.
You C, Li G, Zhang Y, Zhang X, Shan H, Li M, et al. CT super-resolution GAN constrained by the identical, residual, and cycle learning ensemble (GAN-CIRCLE). IEEE Trans Med Imaging. 2019;39(1):188–203.
Chen H, Zhang Y, Zhang W, Liao P, Li K, Zhou J, et al. Low-dose CT via convolutional neural network. Biomed Opt Express. 2017;8(2):679–94.
Chen H, Zhang Y, Kalra MK, Lin F, Chen Y, Liao P, et al. Low-dose CT with a residual encoder-decoder convolutional neural network. IEEE Trans Med Imaging. 2017;36(12):2524–35.
Yang Q, Yan P, Zhang Y, Yu H, Shi Y, Mou X, et al. Low-dose CT image denoising using a generative adversarial network with Wasserstein distance and perceptual loss. IEEE Trans Med Imaging. 2018;37(6):1348–57.
Tang C, Li J, Wang L, Li Z, Jiang L, Cai A, et al. Unpaired low-dose CT denoising network based on cycle-consistent generative adversarial network with prior image information. Computational and mathematical methods in medicine. 2019;2019.
Kang E, Koo HJ, Yang DH, Seo JB, Ye JC. Cycle-consistent adversarial denoising network for multiphase coronary CT angiography. Med Phys. 2019;46(2):550–62.
Liu F, Jang H, Kijowski R, Bradshaw T, McMillan AB. Deep learning MR imaging–based attenuation correction for PET/MR imaging. Radiology. 2018;286(2):676–84.
Ben-Cohen A, Klang E, Raskin SP, Soffer S, Ben-Haim S, Konen E, et al. Cross-modality synthesis from CT to PET using FCN and GAN networks for improved automated lesion detection. Eng Appl Artif Intell. 2019;78:186–94.
Dar SU, Yurt M, Karacan L, Erdem A, Erdem E, Çukur T. Image synthesis in multi-contrast MRI with conditional generative adversarial networks. IEEE Trans Med Imaging. 2019;38(10):2375–88.
Ronneberger O, Fischer P, Brox T. U-net: convolutional networks for biomedical image segmentation. In:International Conference on Medical image computing and computer-assisted intervention: Springer, 2015; 234–41.
Jans LB, Chen M, Elewaut D, Van den Bosch F, Carron P, Jacques P, et al. MRI-based synthetic CT in the detection of structural lesions in patients with suspected sacroiliitis: comparison with MRI. Radiology. 2020:201537.
Guirguis A, Polster J, Karim W, Obuchowski N, Rosneck J, Goodwin R, et al. Interchangeability of CT and 3D “pseudo-CT” MRI for preoperative planning in patients with femoroacetabular impingement. Skeletal Radiol. 2020;49(7):1073–80.
Hiasa Y, Otake Y, Takao M, Matsuoka T, Takashima K, Carass A, et al. Cross-modality image synthesis from unpaired data using CycleGAN. In:International Workshop on Simulation and Synthesis in Medical Imaging: Springer, 2018; 31–41.
Lee JH, Han IH, Kim DH, Yu S, Lee IS, Song YS, et al. Spine computed tomography to magnetic resonance image synthesis using generative adversarial networks: a preliminary study. Journal of Korean Neurosurgical Society. 2020;63(3):386.
Liu F. SUSAN: segment unannotated image structure using adversarial network. Magn Reson Med. 2019;81(5):3330–45.
Galbusera F, Niemeyer F, Seyfried M, Bassani T, Casaroli G, Kienle A, et al. Exploring the potential of generative adversarial networks for synthesizing radiological images of the spine to be used in in silico trials. Frontiers in bioengineering and biotechnology. 2018;6:53.
Cronin NJ, Finni T, Seynnes O. Using deep learning to generate synthetic B-mode musculoskeletal ultrasound images. Computer Methods and Programs in Biomedicine. 2020:105583.
Cuadra MB, Favre J, Omoumi P. Quantification in musculoskeletal imaging using computational analysis and machine learning: segmentation and radiomics. Seminars in Musculoskeletal Radiology. 2020;24(1):50–64.
Buckler AJ, Bresolin L, Dunnick NR, Sullivan DC. A collaborative enterprise for multi-stakeholder participation in the advancement of quantitative imaging. Radiology. 2011;258(3):906–14.
Galbusera F, Casaroli G, Bassani TJJS. Artificial intelligence and machine learning in spine research. 2019;2(1):e1044.
Glocker B, Feulner J, Criminisi A, Haynor DR, Konukoglu E. Automatic localization and identification of vertebrae in arbitrary field-of-view CT scans. In:International Conference on Medical Image Computing and Computer-Assisted Intervention: Springer, 2012; 590–8.
Chen H, Shen C, Qin J, Ni D, Shi L, Cheng JC, et al. Automatic localization and identification of vertebrae in spine CT via a joint learning model with deep neural networks. In:International conference on medical image computing and computer-assisted intervention: Springer, 2015; 515–22.
Yang D, Xiong T, Xu D, Zhou SK, Xu Z, Chen M, et al. Deep image-to-image recurrent network with shape basis learning for automatic vertebra labeling in large-scale 3D CT volumes. In:International Conference on Medical Image Computing and Computer-Assisted Intervention: Springer, 2017; 498–506.
Liao H, Mesfin A, Luo J. Joint vertebrae identification and localization in spinal CT images by combining short-and long-range contextual information. IEEE Trans Med Imaging. 2018;37(5):1266–75.
Lu J-T, Pedemonte S, Bizzo B, Doyle S, Andriole KP, Michalski MH, et al. Deepspine: automated lumbar vertebral segmentation, disc-level designation, and spinal stenosis grading using deep learning. 2018.
Lessmann N, Van Ginneken B, De Jong PA, Išgum I. Iterative fully convolutional neural networks for automatic vertebra segmentation and identification. Med Image Anal. 2019;53:142–55.
Chang PD, Wong TT, Rasiej MJ. Deep learning for detection of complete anterior cruciate ligament tear. J Digit Imaging. 2019;32(6):980–6.
Liu F, Guan B, Zhou Z, Samsonov A, Rosas H, Lian K, et al. Fully automated diagnosis of anterior cruciate ligament tears on knee MR images by using deep learning. Radiology: Artificial Intelligence. 2019;1(3):180091.
Bien N, Rajpurkar P, Ball RL, Irvin J, Park A, Jones E, et al. Deep-learning-assisted diagnosis for knee magnetic resonance imaging: development and retrospective validation of MRNet. PLoS medicine. 2018;15(11):e1002699.
Liu F, Zhou Z, Samsonov A, Blankenbaker D, Larison W, Kanarek A, et al. Deep learning approach for evaluating knee MR images: achieving high diagnostic performance for cartilage lesion detection. Radiology. 2018;289(1):160–9.
Zhou Z, Zhao G, Kijowski R, Liu F. Deep convolutional neural network for segmentation of knee joint anatomy. Magn Reson Med. 2018;80(6):2759–70.
Nelson AE. How feasible is the stratification of osteoarthritis phenotypes by means of artificial intelligence? In: Taylor & Francis, 2020.
Tolpadi AA, Lee JJ, Pedoia V, Majumdar S. Deep learning predicts total knee replacement from magnetic resonance images. Sci Rep. 2020;10(1):6371.
Tiulpin A, Thevenot J, Rahtu E, Lehenkari P, Saarakkala S. Automatic knee osteoarthritis diagnosis from plain radiographs: a deep learning-based approach. Sci Rep. 2018;8(1):1–10.
Górriz M, Antony J, McGuinness K, Giró-i-Nieto X, O’Connor NE. Assessing knee OA severity with CNN attention-based end-to-end architectures. arXiv preprint arXiv:1908.08856. 2019.
Pedoia V, Norman B, Mehany SN, Bucknor MD, Link TM, Majumdar S. 3D convolutional neural networks for detection and severity staging of meniscus and PFJ cartilage morphological degenerative changes in osteoarthritis and anterior cruciate ligament subjects. J Magn Reson Imaging. 2019;49(2):400–10.
Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16(12):1433–41.
Segal NA, Nevitt MC, Gross KD, Hietpas J, Glass NA, Lewis CE, et al. The Multicenter Osteoarthritis Study (MOST): opportunities for rehabilitation research. PM & R: the journal of injury, function, and rehabilitation. 2013;5(8).
Heimann T, Morrison BJ, Styner MA, Niethammer M, Warfield S. Segmentation of knee images: a grand challenge. In:Proc. MICCAI Workshop on Medical Image Analysis for the Clinic, 2010; 207–14.
Astuto B, Flament I, N KN, Shah R, Bharadwaj U, T ML, et al. Automatic deep learning-assisted detection and grading of abnormalities in knee MRI studies. Radiol Artif Intell. 2021;3(3):e200165.
Gan H-S, Ramlee MH, Wahab AA, Lee Y-S, Shimizu A. From classical to deep learning: review on cartilage and bone segmentation techniques in knee osteoarthritis research. Artificial Intelligence Review. 2020:1–50.
Norman B, Pedoia V, Majumdar S. Use of 2D U-Net convolutional neural networks for automated cartilage and meniscus segmentation of knee MR imaging data to determine relaxometry and morphometry. Radiology. 2018;288(1):177–85.
Cheng R, Alexandridi NA, Smith RM, Shen A, Gandler W, McCreedy E, et al. Fully automated patellofemoral MRI segmentation using holistically nested networks: implications for evaluating patellofemoral osteoarthritis, pain, injury, pathology, and adolescent development. Magn Reson Med. 2020;83(1):139–53.
Pinto A, Reginelli A, Pinto F, Lo Re G, Midiri F, Muzj C, et al. Errors in imaging patients in the emergency setting. Br J Radiol. 2016;89(1061):20150914.
Pinto A, Berritto D, Russo A, Riccitiello F, Caruso M, Belfiore MP, et al. Traumatic fractures in adults: missed diagnosis on plain radiographs in the Emergency Department. Acta Bio Medica: Atenei Parmensis. 2018;89(Suppl 1):111.
Ren S, He K, Girshick R, Sun J. Faster r-cnn: towards real-time object detection with region proposal networks. IEEE Trans Pattern Anal Mach Intell. 2016;39(6):1137–49.
Ghiasi G, Lin T-Y, Le QV. Nas-fpn: learning scalable feature pyramid architecture for object detection. In:Proceedings of the IEEE conference on computer vision and pattern recognition, 2019; 7036–45.
Olczak J, Fahlberg N, Maki A, Razavian AS, Jilert A, Stark A, et al. Artificial intelligence for analyzing orthopedic trauma radiographs: deep learning algorithms—are they on par with humans for diagnosing fractures? Acta Orthop. 2017;88(6):581–6.
Kim D, MacKinnon T. Artificial intelligence in fracture detection: transfer learning from deep convolutional neural networks. Clin Radiol. 2018;73(5):439–45.
Tomita N, Cheung YY, Hassanpour S. Deep neural networks for automatic detection of osteoporotic vertebral fractures on CT scans. Comput Biol Med. 2018;98:8–15.
Jones RM, Sharma A, Hotchkiss R, Sperling JW, Hamburger J, Ledig C, et al. Assessment of a deep-learning system for fracture detection in musculoskeletal radiographs. NPJ digital medicine. 2020;3(1):1–6.
Szegedy C, Vanhoucke V, Ioffe S, Shlens J, Wojna Z. Rethinking the inception architecture for computer vision. In:Proceedings of the IEEE conference on computer vision and pattern recognition, 2016; 2818–26.
Russakovsky O, Deng J, Su H, Krause J, Satheesh S, Ma S, et al. Imagenet large scale visual recognition challenge. Int J Comput Vision. 2015;115(3):211–52.
Funding
This work was supported by a National Research Foundation (NRF) grant funded by the Korean government, Ministry of Science and ICT (MSIP, 2018R1A2B6009076).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shin, Y., Kim, S. & Lee, Y. AI musculoskeletal clinical applications: how can AI increase my day-to-day efficiency?. Skeletal Radiol 51, 293–304 (2022). https://doi.org/10.1007/s00256-021-03876-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-021-03876-8