Abstract
Necrotizing fasciitis (NF) is a rare, life-threatening soft-tissue infection and a medical and surgical emergency, with increasing incidence in the last few years. It is characterized by a rapidly spreading, progressive necrosis of the deep fascia and subcutaneous tissue. Necrotizing fasciitis is often underestimated because of the lack of specific clinical findings in the initial stages of the disease. Many adjuncts such as laboratory findings, bedside tests—e.g., the “finger test” or biopsy—and imaging tests have been described as being helpful in the early recognition of the disease. Imaging is very useful to confirm the diagnosis, but also to assess the extent of the disorder, the potential surgical planning, and the detection of underlying etiologies. The presence of gas within the necrotized fasciae is characteristic, but may be lacking. The main finding is thickening of the deep fasciae due to fluid accumulation and reactive hyperemia, best seen on magnetic resonance imaging.
Similar content being viewed by others
Introduction
Necrotizing fasciitis (NF) is an uncommon and aggressive soft-tissue infection, usually caused by toxin-producing, virulent bacteria. It involves the deep fascia and is characterized by the extensive deterioration of the surrounding tissue [1]. It is often associated with severe systemic toxicity and is usually rapidly fatal, unless promptly recognized and aggressively treated [2–7]. NF can be initially difficult to differentiate from cellulitis and other superficial infections of the skin. In fact, only 15 to 34 % of patients with NF have an accurate diagnosis at admission [7–9]. Only early diagnosis and aggressive surgical treatment can reduce mortality and morbidity [7, 10, 11].
Despite many advances in the understanding of this disease and great improvements in medical care, the mortality has not changed in the last 30 years and remains as high as 70 % [7, 12, 13]. The purpose of this article is to review of the pathophysiology and diagnosis of NF, stressing the role of imaging in the early diagnosis of the disease.
Terminology and historical background
Necrotizing fasciitis is a rare and fatal soft tissue infection primarily involving the fascia and extending to the neighboring tissues, causing necrosis and systemic failure [2, 14]. This disease has concerned physicians for centuries. It was first described by Hippocrates in the 5th century BC. In 1952, Wilson coined the term “necrotizing fasciitis,” which is still used nowadays [2, 10, 15].
The recent tendency is to encompass all necrotizing infection regardless of the depth of infection in the term “necrotizing soft tissue infections” (NSTI). All these soft tissue infections involve a similar approach to diagnosis and treatment regardless of their anatomical location and depth of infection [3, 15–18].
Anatomy
Certain anatomical considerations are important to understand the pathophysiology of NSTI. Most bacteria and fungi can multiply within viable tissue, but fibrous attachments or “boundaries” between subcutaneous tissues and fascia (e.g., scalp, hands) can help limit the spread of infection. The natural lack of fibrous attachments in the larger areas of the body (e.g., trunk, extremities) facilitates widespread infection [18].
From the surface down and forming concentric circles, we find the skin (epidermis and dermis), superficial fascia or subcutaneous tissue (hypodermis), the deep fascia, and muscles. The deep fascia continues with the epimysium (connective tissue surrounding muscles), and sends prolongations (intermuscular septa) that divide the different muscle compartments [1, 19]. Since the fascia is a continuum from the surface to the endomysium muscles, it is the route by which a surface process spreads to muscles or bones and vice versa (Fig. 1) [7, 20–22].
Epidemiology and risk factors
Necrotizing fasciitis is a rare disease. The incidence of NF has been reported to be 0.4 cases per 100,000 inhabitants in the United States (USA), while in Western Europe it is about 1 case per 100,000 inhabitants [5, 13, 23]. This disease is predominantly seen in adult patients, with incidence increasing progressively with age [3], reaching 12 per 100,000 in subjects over the age of 80. In children, the reported rate is 0.08 per 100,000 [11, 24]. The difference in incidence observed between men and women varies from 1:1 [23] to 3:1. The latter is probably justified by the predominantly masculine incidence of Fournier’s gangrene [3]. The incidence has increased since 1980, although the exact reason remains speculative. Possible explanations include increased microbial virulence and resistance due to the excessive use of antibiotics, population aging, increased rates of immunosuppression, and better disease reporting [16, 23].
While the understanding of the pathophysiology of NF continues to improve, its mortality rate remains alarmingly high, reaching up to 70 %, mainly due to delayed diagnosis and, consequently, delayed operative debridement [3, 10, 16, 25] .
More than half of patients with NSTI have pre-existing conditions that render them susceptible to infections, such as obesity, diabetes mellitus, chronic renal failure, peripheral vascular disease, drug misuse, alcohol abuse, liver disease, malignancy, and chemotherapy, conditions that are very frequent in the Western world nowadays [2, 3, 7, 12, 18, 23, 26]. Risk factors in the pediatric population include malnutrition, skin infections, sepsis, and immunosuppression [7, 23]. NSTI, however, can also occur in otherwise “healthy adults” (i.e., few or no comorbidities); hence, it is impossible to predict which patients are at risk of NSTI. In almost all cases, there is a precipitating event such as a history of surgery or a penetrating injury that can be as trivial as an insect bite or a scratch (Table 1) [3, 5, 7, 23, 26].
Microbiology: classification according to etiology
Four basic causes of microbial subtypes are described. The two more extensive causes are type I, or polymicrobial, and type II, or monomicrobial.
Type I is the most common one (55–80 %), with an average of four pathogens, usually a mixture of aerobic and anaerobic organisms, a combination of Gram-positive cocci, such as S. aureus, S. pyogenes, and enterococci, Gram-negative rods such as E. coli and P. aeruginosa, and anaerobes like Bacteroides or Clostridium species. Despite its historical prevalence, C .perfringens is nowadays a rare cause owing to improvements in sanitation and hygiene. If they occur, they typically involve the perineal and trunk areas of immunocompromised patients, particularly in diabetics [7, 8, 16, 23, 26].
Type II is a monomicrobial infection that tends to occur in healthy young immunocompetent hosts with a history of recent trauma, usually trivial, often localized to the extremities. Group A (Streptococcus pyogenes), alone or in association with S. aureus, is the typical pathogen. It can be associated with streptococcal toxic shock syndrome (STSS), an exotoxin-driven disease that significantly increases the mortality from <40 to 67 % with up to half of patients needing amputation. Community-acquired methicillin-resistant S. aureus (MRSA) has increasingly been described in NF, in as many as one-third of cases [3, 8, 10, 12, 16, 18, 27].
The infections caused by Vibrio species are classified as type III or hyper-acute type with a fulminant systemic course with no epidermal manifestations. It can be acquired through a skin lesion and exposure to warm sea water. Another known important risk factor is moderate or severe liver disease [3, 10, 12, 16, 27, 28]
Finally, some authors have described type IV, or fungal infection (Table 2) [3, 18, 27, 29].
Pathophysiology
Knowledge of the pathophysiology is essential to the understanding of the clinical course and the importance of an early diagnosis to the prognosis of the disease. Microbial invasion of the subcutaneous tissues (SCT) occurs either through external trauma or from direct spread from perforated viscera or a urogenital organ. After that, microorganisms proliferate and generate toxins and enzymes like hyaluronidase, enabling horizontal extension through deep fascial planes. As this process progresses, thrombosis of the perforating nutrient vessels causes progressive dermis and skin ischemia, leading to bullae formation, ulceration, and skin necrosis. Ischemia and necrosis generate an inflammatory response and cytokine liberation, producing a systemic response that can lead to multi-organ failure (Fig. 2) [3, 10, 16, 25].
Diagnosis
Establishing the diagnosis of NSTI is challenging. As stated before, early diagnosis results in early therapy and decreased mortality. Early diagnosis is missed in as many as 85–100 % of cases [23]. Clinically, early necrotizing fasciitis can be easily misinterpreted as noncomplicated cellulitis or abscess. The first and most important consideration for an accurate, prompt diagnosis is to have a high index of suspicion [10, 15, 16, 18, 23].
Clinical findings
Necrotizing fasciitis typically presents with patchy discoloration of the skin, severe pain, and ill-defined swelling. Certain patients, notably those with diabetic neuropathy, may experience minimal pain, which can render the diagnosis of NF even more challenging. There is a fast progression to tense edema, grayish-brown discharge, vesicles, bullae, necrosis, and crepitus [3, 7, 10, 15, 18, 23, 30]. The latter is a later sign, very specific, but found in only 13–31 % of patients [7, 16, 30]. In advanced phases, anesthesia over the site of the erythema may occur [3, 7]. Muscle hypoxia and swelling alter oxygen tension, increasing intracompartmental pressure, and sometimes resulting in compartment syndrome [27]. While inflammatory systemic signs and symptoms like fever, dehydration, confusion, and tachycardia [3, 7, 15, 16, 26] can occur, patients may appear quite well, at least initially. Indeed, 53 % of the patients are febrile and only 18 % are hypointense at presentation. This is particularly the case in immunosuppressed patients [10]. Even in the most experienced hands, clinical findings are not accurate enough for diagnosis. Therefore, they need to be combined with other diagnostic data like laboratory, and especially imaging findings [3, 10, 16].
Laboratory findings
Even though laboratory findings are not specific, leukocytosis with neutrophilia, acidosis, altered coagulation profile, impaired renal function, and increased creatinine kinase and inflammatory markers, are helpful if viewed within the whole clinical context [3, 7, 10, 15, 16, 18, 23, 25, 30]. The combination of certain laboratory tests may enable discrimination between NSTI and non-necrotizing infections:
-
As found by Wall et al., the combination of a white blood cell (WBC) count >15,400 cells/ml and a serum-sodium (Na+) level <135 mmol/l shows a negative predictive value (NPV) of 99 % and a positive predictive value (PPV) of 26 % for NSTI [31].
-
Wong et al. proposed a scoring system (laboratory risk indicator for necrotizing fasciitis [LRINEC]) based on six different variables that give a specific number of points (Table 3). The total score ranges from 0 to 14 and classifies patients into three categories: low, intermediate, and high risk of having NF [25, 32]. Some other retrospective studies support this score, and relate it to increased rates of mortality and amputation in patients with LRINEC score ≥6. This score, however, needs to be prospectively validated [3, 5, 7, 10, 15, 16, 23, 33]
Bedside tests, surgical exploration, and pathology
The gold standard modality for the diagnosis of NSTI remains operative exploration, and it is also the mandatory live-saving treatment. In doubtful cases, a “finger test” and frozen biopsy have been used as complementary diagnostic modalities. Once NSTI is confirmed, the incision is extended and additional debridement is performed [5, 10, 15, 16, 23, 30].
The “finger test” is a bedside procedure under local anesthesia in which a 2-cm incision is made down to the deep fascia. Lack of bleeding, presence of smelly “dishwater pus”, noncontracting muscles, and lack of tissue resistance to blunt finger dissection indicates NF [5, 10, 15, 16, 23].
Tissue specimens for culture and histology are crucial [10]. The specimens should be generous and taken from the interface between live and dead tissue [10, 16, 23]. Histological criteria for diagnosing NF are necrosis of the deep fascia, polymorphonuclear infiltration of the dermis and fascia, fibrin thrombi with fibrinoid necrosis of arterial and venous walls of arteries and veins coursing through the fascia and the presence of microorganisms within the destroyed fascia and dermis [2, 5, 10, 15, 16].
Imaging tests
Imaging tests are an invaluable diagnostic adjunct. They may confirm the diagnosis in cases where signs are unclear, delineate the extent of the disease, and help in the identification of the source and complications of the infection. They also bring additional anatomical information for surgical planning. Anyhow, imaging should never delay operative debridement. Plain films, ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI) may be used, but the latter is the recommended modality [1, 2, 10, 14, 15, 18].
Plain radiography
Most radiographic findings are similar to those for cellulitis, with increased soft-tissue thickness and opacity. Frequently, films are unremarkable until the infection and necrosis are advanced and subcutaneous air can be identified. The latter is a very specific sign of NSTI, but is not very sensitive (Fig. 3) [10, 14, 15]. Radiographic evidence of air in the soft tissue may be present before clinical crepitus is detected [34]. This test can also provide additional information, such as the presence of a radiopaque foreign body like a surgical device [35]
Ultrasound
Ultrasound, a convenient and non-invasive tool, has been widely used to evaluate patients in the emergency care unit. As NF is many times misdiagnosed as cellulitis or deep venous thrombosis; an ultrasound is often ordered to rule out the latter [35].
Suggestive ultrasound features are thickening and distortion of the deep fascia (>4 mm; fasciitis), turbid fluid collections along the deep fascia, features of myositis, i.e., muscle hypo- or hyperechogenic swelling, findings of cellulitis, i.e., swelling of the subcutaneous tissue, including cobblestone appearance (hyperechogenic subcutaneous tissue traversed by hypoechoic strands of fluid). Increased Doppler hyperemia or thrombosed small blood vessels [36, 37] can also be seen. Based on these criteria, a 62-patient prospective observational study found a PPV of 83 % and a NPV of 95.4 % for the diagnosis of NF [37].
The examination may be limited by soft tissue gas, although this finding may be of diagnostic benefit [14].
Assessing for the patency of the underlying vessels is critical in the early detection of compartment syndrome, a severe complication that can result in the loss of the affected extremity, and this assessment can be effectively accomplished using Doppler ultrasound [35].
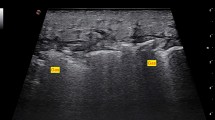
In the case of Fournier gangrene, the main ultrasound findings are a thickened edematous scrotal wall that may contain hyperechoic foci with reverberation artifacts (gas). The testes and epididymis are often normal in size and echotexture owing to their direct aorta branch blood supply (Fig. 4) [34].
Ultrasound images of a 63-year-old man with history of lung cancer who presented with scrotal crepitus. Findings of Fournier gangrene were seen and surgically confirmed. a Thickened edematous scrotal wall containing hyperechoic foci with reverberation artifacts related to gas (arrow). b Normal testicles. c Contrast-enhanced axial CT of the same patient showing gas (arrow) in the gluteal and right inguinal region
Ultrasound has been most useful in the pediatric age group [14]. Its role in managing NSTI, however, is still unclear. Moreover, it may underestimate the extent of the disease in part because the infiltration of the hypodermis blocks ultrasound transmission [1]. Although it is not the first-line recommended test, the portability of the equipment provides an advantage, especially when an image-guided intervention is needed [35].
Computed tomography
Computed tomography is a quick and accessible tool for NSTI diagnosis. It is the most sensitive imaging modality for the detection of subtle soft tissue air collections. It plays a vital role in rapidly suggesting the diagnosis [20, 35]. CT characteristics correlate with the pathological findings of liquefied, necrotic tissue and inflammation resulting from bacterial exotoxins released in the fascial layers [14].
The CT hallmarks of NF are edema and asymmetrical thickening with or without enhancement of the deep fascial layers (fasciitis), stranding of the fat of subcutaneous tissue (cellulitis), fluid collections along the fascial planes, and enhancement and thickening of regional muscles (myositis), associated or not with foci of air within the soft tissue (Fig. 5) [14, 38]. The presence of gas in the subcutaneous tissue caused by gas-forming anaerobic organisms is a specific and hallmark sign of NF. Such a finding is seen in 10–55 % in some series (Fig. 6) [7, 16, 20, 39].
Axial images of a contrast-enhanced cervical CT of a 55-year-old diabetic woman with a recent history of dental infection. Findings corresponding to necrotizing fasciitis were seen and surgically confirmed. a Stranding of the fat of the subcutaneous tissue and skin thickening (cellulitis; asterisk), thickening and blurring of the left sternocleidomastoid and platysma muscles (myositis; thick arrow). b Asymmetrical thickening of the superficial and deep fascial layers (fasciitis; thin arrow) and stranding of the fat of the subcutaneous tissue and skin thickening (cellulitis; asterisk). An endotracheal tube was in place
A 75-year-old male patient with a history of diabetes with NF in the right thigh. Axial images of a CT of a the pelvis and b the lower extremities show stranding of the fat of the subcutaneous tissue, thickening of the skin (cellulitis), and thickening of the superficial and deep fascial layers (fasciitis; asterisk). Subcutaneous emphysema (thick arrow) is also noted. A continuity solution in the right gluteus is seen, indicating a decubitus ulcer (thin arrow)
While fascial fluid collections are typically non-focal; abscesses may be seen [14].
Differentiation of NSTI from cellulitis or postoperative changes is difficult. Typically, in NF there is involvement of fascial planes and the sparing of the superficial epidermis. Postoperative changes are more difficult to differentiate since they may include fluid tracking along fascial planes and subcutaneous emphysema. Serial CT examinations should show steady resolution in the absence of infection or other complicating factors [40].
Potential advantages of CT include the ability to delineate the disease location and extent [40], or detect underlying infectious sources such a diverticulitis and intestinal perforation (Fig.7) [39]. CT may also demonstrate serious complications such as vascular thrombosis or vascular rupture complicating the tissue necrosis [14].
Fournier gangrene after rectal perforation in a 60-year-old man with a history of rectal carcinoma. a Axial unenhanced CT and b coronal multiplanar reconstruction from a CT abdomen show rectal perforation (thin arrow) and subcutaneous emphysema in the perineal and right gluteal regions (thick arrow)
Special consideration of NF of the head and neck must be taken into account. Although uncommon, it can lead to descending necrotizing mediastinitis (DNM) with a higher mortality rate [38, 41, 42]. Since associated mediastinitis is often present from the beginning, CT examination should include the chest (Fig. 8) [38].
A 33-year-old male patient with NF and descending necrotizing mediastinitis (DNM). a, b Ultrasound images of the cervical region show turbid fluid collections along the deep fascia (asterisk). c, d Axial images from a CT neck and chest with contrast agent show fluid collections within the pretracheal visceral space and within the superior and anterior mediastinum (asterisk). Stranding of the fat of the subcutaneous tissue (cellulitis; thin red arrow) and the thickening and blurring of the bilateral sternocleidomastoid and platysma muscles (myositis; thick arrow) are also noted
Some known causes are dental infections, peritonsillar and pharyngeal abscesses, insect stings, neck or dental surgery or foreign bodies [27, 43]
Magnetic resonance imaging
Magnetic resonance imaging is the recommended imaging modality, as it provides excellent soft-tissue definition and multiplanar capabilities [1, 44]. It is an invaluable diagnostic adjunct, but it may not be readily available and is certainly not cheap [10]. The sensitivity and specificity of MRI in the detection of NF has been reported to be 89 to 100 % and 46 to 86 % respectively in different studies [25, 45–47].
Some authors have described features that they believe are specific to NF. These include hyperintense signal in subcutaneous tissue in fluid-sensitive sequences (cellulitis), deep fascial thickening (fasciitis), deep fascial fluid collections, and hyperintense T2 signal within the muscles (myositis; Fig. 9) [10, 44–48]. The involvement of three or more compartments in one extremity has also been described as an NSTI indicator (Fig. 10) [47]. Gas in the deep fascial planes is inconstant and characterized by signal voids, is best seen on gradient echo sequences, and is a hallmark of NF [35, 44, 45, 47].
A 33--year-old, previously healthy man, with NF following left thigh trauma. a Fat-suppressed T2-weighted axial image shows areas of high signal intensity in nearly all fasciae surrounding the muscles of the antero-medial and posterior compartments (fasciitis ± fluid collections; thin arrows), hyperintensity in the subcutaneous tissue (cellulitis; asterisk) and within the muscles (myositis; thick arrow). b Fat-suppressed T1-weighted axial image shows the same findings as T2, stranding of subcutaneous tissue (cellulitis) and the thickness of the deep fasciae (fasciitis and fluid collections). c Post-contrast fat-suppressed T1-weighted axial image and d post-contrast subtraction T1-weighted axial image show enhancement in some of the hyperintense fasciae seen in a (arrows)
A 30-year-old man with NF following a penetrating injury to the right elbow. a Fat-suppressed axial T2-weighted image shows areas of high signal intensity in the deep fasciae surrounding muscle of the lateral, posterior, and medial compartments of the right arm (fasciitis and collections; arrows), as well as areas of high signal intensity in the subcutaneous tissue (cellulitis; asterisk). b, c Unenhanced T1-weighted axial image and post-contrast fat-suppressed T1-weighted image show enhancement within the subcutaneous tissue (asterisk) as well as in some of the hyperintense fasciae seen in a (arrows)
A thick (>3 mm) abnormal perifascial signal hyperintensity on fat-suppressed (FS) T2-weighted images is seen more frequently in NSTI and explained as purulent perifascial fluid and edema [47]. This feature, independent of the thickness, has been described as a sensitive, albeit nonspecific, feature [43, 44].
Fascial enhancement and the lack of enhancement of nearby areas with increased signal intensity on T2-weighted sequences have both been described as reliable indicators for NSTI [10, 14, 44, 45, 47, 49]. Some explanations have been proposed. Contrast enhancement in deep tissue areas is often indicative of major damage to the neighboring capillary network with extravasations of contrast material and does not reflect hyperemia (Fig. 11). As necrosis progresses, diminished or no enhancement of the fascia is then observed. This is associated with advanced necrosis and an underestimation of the tissue involved. An abscess-like delineation of the necrotic tissue with rim enhancement has also been observed [44, 45, 47].
An 80-year-old man with NF following trauma in the right thigh. a Fat-suppressed T2-weighted axial image shows areas of high signal intensity in nearly all fasciae surrounding the muscles of the antero-medial, posterior, and lateral compartments (fasciitis ± fluid collections; thin arrows), hyperintensity in the subcutaneous tissue (cellulitis; asterisk) and within the muscles (myositis; thick arrow). b Post-contrast fat-suppressed T1-weighted axial image shows ring-like enhancement in the muscles of the medial compartment (thick arrow) with no enhancement in the deep fasciae (thin arrows)
A peripheral band-like hyperintense signal in muscles on the FS T2-weighted images and on FS contrast-enhanced (CE) T1-weighted images has been described as a typical feature of NF and also as a possible tool for differentiating NF from pyomyositis (diffuse hyperintense FST2 and FS CE T1) (Figs. 12, 13) [46]. In another study, this band-like hyperintense signal has been described in necrotizing and non-necrotizing pathological conditions, and is explained as being a reactive change in muscle due to the inflammation of adjacent fascia with or without necrosis [47].
A 48-year-old man, ex-intravenous drug user, with NF, muscular necrosis, and compartment syndrome. a Post-contrast axial CT image showing no muscular enhancement of the right arm and a hyperdense area in the postero-lateral compartment corresponding with hemorrhage (curved arrow). b T2-weighted axial image showing areas of high signal intensity in nearly all fasciae surrounding the muscles of the antero-medial compartments (fasciitis ± fluid collections; thin arrows), hyperintensity in the subcutaneous tissue (cellulitis; asterisk) and within the muscles (myositis; thick arrow). c Fat-suppressed T1-weighted axial image shows a hyperintense area in the postero-lateral compartment (same finding as in a) corresponding with hemorrhage (curved arrow) d Post-contrast fat-suppressed T1-weighted axial image shows marked heterogeneous enhancement in the fasciae (arrows) and muscles (thick arrow) of the antero-medial compartment due to muscular necrosis
A 75-year-old diabetic man with left neck paravertebral myonecrosis, C6 osteomyelitis, and epidural abscess. a Plain antero-posterior neck radiography showing left paravertebral increased soft-tissue thickness and opacity (asterisk) b, c Post-contrast axial neck CTs show heterogeneous thickness of the left paravertebral musculature, C6 left transverse process erosion (triangle), and the presence of an air bubble (curved arrow) at an upper cervical level. d T2-weighted axial image shows C6 left transverse process edema (triangle); thickness and heterogeneous hyperintensity of the left paravertebral musculature (myositis; thick arrow). e, f Post-contrast fat-suppressed T1-weighted axial images at C6 level and upper level showing left epidural abscess (arrowhead), deep fascial enhancement (thin arrow), stranding of subcutaneous tissue (cellulitis; asterisk), C6 left transverse process erosion (triangle) and heterogeneous muscular enhancement (myonecrosis; thick arrow). Air bubbles are also visualized (curved arrow)
Fluid-sensitive sequences may overestimate the process secondary to adjacent reactive edema; in contrast, the degree of contrast enhancement may result in an underestimation of the extent of disease secondary to hypoperfusion and tissue necrosis [10, 14, 35, 47].
The sensitivity of MRI often exceeds its specificity [10]. Fascial thickening and high signal intensity on T2-weighted images is not specific to NSTI. This has also been reported in patients with non-infectious inflammatory eosinophilic fasciitis, rheumatic diseases, phlebedema, lymphedema, exertional muscle injury, pyomyositis, and neoplastic diseases. They may have similar MRI findings, but most of them can be diagnosed from their specific clinical findings and history [48, 49].
What nearly everyone agrees upon is that negative deep fascial involvement on MRI effectively excludes NF [10].
Management
Surgical treatment consistent with fasciotomy and debridement of the necrotic tissue is mandatory [14]. Boundaries of the incision must be at least as wide as the rim of the cellulitis and should comprise healthy, bleeding tissue [16]. Intensive care, including fluid resuscitation, pressor support, cardiac monitoring, and ventilator support are usually necessary [14]. Broad-spectrum intravenous antibiotic therapy should be maintained until the causative organisms have been identified [14, 16, 23]. Other additional therapies such as intravenous immunoglobulin and post-surgery hyperbaric oxygen can be used [16, 23, 27].
After this extensive revision, we find necessary to create a diagnostic algorithm that, far from being a validated clinical guide, may help to achieve a prompt diagnosis and thus facilitate management of individual patients (Fig. 14).
Diagram showing NF diagnostic algorithm. If we have a clinical suspicion of NF, imaging, bedside, and laboratory testing may be down as diagnostic adjuncts in order to achieve a correct and prompt treatment. If no deep fascial involvement is seen on MRI, NF can almost be excluded. Although the laboratory risk indicator for NF (LRINEC) is not completely validated, a score ≤5 indicates a low risk of NF (<50 % probability) and a score ≥6 indicates an intermediate to high risk. WBC white blood cells [25, 32]
Conclusion
Necrotizing fasciitis is an uncommon, rapidly progressive, and highly lethal disease with a poor prognosis unless promptly treated [14, 23]. High-risk patient populations do exist, but healthy young patients are also susceptible [16]. A high index of suspicion is needed to ensure a timely intervention [23]. Characteristic clinical presentations like skin necrosis, greyish discharge or anesthesia are only observable later, while initial findings may be misleading (rubor, pain, and edema). Some known diagnosis adjuncts include laboratory, bedside, and imaging tests [10, 14, 16, 23]. They may confirm or exclude diagnosis, but should never delay emergency surgical treatment. MRI is the recommended imaging modality if available, but CT or ultrasound is also useful. Findings including cellulitis, fasciitis, myositis, and fluid collections confirm diagnosis (Table 4). The presence of gas within the necrotized fasciae is characteristic, but may be lacking. Negative deep fascial involvement effectively excludes NF [1, 10].
References
Malghem J, Lecouvet FE, Omoumi P, Maldague BE, Vande Berg BC. Necrotizing fasciitis: contribution and limitations of diagnostic imaging. Joint Bone Spine. 2013;80(2):146–54.
Green RJ. Necrotizing fasciitis. Chest. 1996;110(1):219.
Roje Z, Roje Z, Matic D, Librenjak D, Dokuzovic S, Varvodic J. Necrotizing fasciitis: literature review of contemporary strategies for diagnosing and management with three case reports: torso, abdominal wall, upper and lower limbs. World J Emerg Surg. 2011;6(1):46.
Espandar R, Sibdari SY, Rafiee E, Yazdanian S. Necrotizing fasciitis of the extremities: a prospective study. Strategies Trauma Limb Reconstr . 2011;6(3):121–5.
Shimizu T, Tokuda Y. Necrotizing fasciitis. Intern Med. 2010;49(12):1051–7.
Mulcahy H, Richardson ML. Imaging of necrotizing fasciitis: self-assessment module. AJR Am J Roentgenol. 2010;195(6 Suppl):S66–9.
Puvanendran R, Huey JC, Pasupathy S. Necrotizing fasciitis. Can Fam Physician. 2009;55(10):981–7.
Wong CH, Chang HC, Pasupathy S, Khin LW, Tan JL, Low CO. Necrotizing fasciitis: clinical presentation, microbiology, and determinants of mortality. J Bone Joint Surg Am. 2003;85-A(8):1454–60.
Hefny AF, Eid HO, Al-Hussona M, Idris KM, Abu-Zidan FM. Necrotizing fasciitis: a challenging diagnosis. Eur J Emerg Med. 2007;14(1):50–2.
Wong CH, Wang YS. The diagnosis of necrotizing fasciitis. Curr Opin Infect Dis. 2005;18(2):101–6.
Legbo JN, Shehu BB. Necrotizing fasciitis: a comparative analysis of 56 cases. J Natl Med Assoc. 2005;97(12):1692–7.
Chen IC, Li WC, Hong YC, Shie SS, Fann WC, Hsiao CT. The microbiological profile and presence of bloodstream infection influence mortality rates in necrotizing fasciitis. Crit Care. 2011;15(3):R152.
Psoinos CM, Flahive JM, Shaw JJ, Li Y, Ng SC, Tseng JF, et al. Contemporary trends in necrotizing soft-tissue infections in the United States. Surgery. 2013;153(6):819–27.
Fugitt JB, Puckett ML, Quigley MM, Kerr SM. Necrotizing fasciitis. Radiographics. 2004;24(5):1472–6.
Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis. 2007;44(5):705–10.
Sarani B, Strong M, Pascual J, Schwab CW. Necrotizing fasciitis: current concepts and review of the literature. J Am Coll Surg. 2009;208(2):279–88.
Gubitosi A, Moccia G, Ruggiero R, Docimo G, Foroni F, Esposito E, et al. Necrotizing soft tissue infections (NSTIs) Literary review and description of a Fournier syndrome case. Ann Ital Chir. 2013;84(1):111–5.
Headley AJ. Necrotizing soft tissue infections: a primary care review. Am Fam Physician. 2003;68(2):323–8.
Lee S, Joo KB, Song SY. Accurate definition of superficial and deep fascia. Radiology. 2011;261(3):994. author reply 994–5.
Fayad LM, Carrino JA, Fishman EK. Musculoskeletal infection: role of CT in the emergency department. Radiographics. 2007;27(6):1723–36.
Kothari NA, Pelchovitz DJ, Meyer JS. Imaging of musculoskeletal infections. Radiol Clin N Am. 2001;39(4):653–71.
Struk DW, Munk PL, Lee MJ, Ho SG, Worsley DF. Imaging of soft tissue infections. Radiol Clin N Am. 2001;39(2):277–303.
Lancerotto L, Tocco I, Salmaso R, Vindigni V, Bassetto F. Necrotizing fasciitis: classification, diagnosis, and management. J Trauma Acute Care Surg. 2012;72(3):560–6.
Legbo JN, Shehu BB. Necrotising fasciitis: experience with 32 children. Ann Trop Paediatr. 2005;25(3):183–9.
Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004;32(7):1535–41.
Hasham S, Matteucci P, Stanley PR, Hart NB. Necrotising fasciitis. BMJ. 2005;330(7495):830–3.
Morgan MS. Diagnosis and management of necrotising fasciitis: a multiparametric approach. J Hosp Infect. 2010;75(4):249–57.
Hau V, Ho CO. Necrotising fasciitis caused by Vibrio vulnificus in the lower limb following exposure to seafood on the hand. Hong Kong Med J. 2011;17(4):335–7.
Buchanan PJ, Mast BA, Lottenberg L, Kim T, Efron PA, Ang DN. Candida albicans necrotizing soft tissue infection: a case report and literature review of fungal necrotizing soft tissue infections. Ann Plast Surg. 2013;70(6):739–41.
Rajan S. Skin and soft-tissue infections: classifying and treating a spectrum. Cleve Clin J Med. 2012;79(1):57–66.
Wall DB, Klein SR, Black S, de Virgilio C. A simple model to help distinguish necrotizing fasciitis from nonnecrotizing soft tissue infection. J Am Coll Surg. 2000;191(3):227–31.
Wong CH, Khin LW. Clinical relevance of the LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score for assessment of early necrotizing fasciitis. Crit Care Med. 2005;33(7):1677.
Wilson MP, Schneir AB. A case of necrotizing fasciitis with a LRINEC score of zero: clinical suspicion should trump scoring systems. J Emerg Med. 2013;44(5):928–31.
Levenson RB, Singh AK, Novelline RA. Fournier gangrene: role of imaging. Radiographics. 2008;28(2):519–28.
Turecki MB, Taljanovic MS, Stubbs AY, Graham AR, Holden DA, Hunter TB, et al. Imaging of musculoskeletal soft tissue infections. Skeletal Radiol. 2010;39(10):957–71.
Jaovisidha S, Leerodjanaprapa P, Chitrapazt N, Nartthanarung A, Subhadrabandhu T, Siriwongpairat P. Emergency ultrasonography in patients with clinically suspected soft tissue infection of the legs. Singapore Med J. 2012;53(4):277–82.
Yen ZS, Wang HP, Ma HM, Chen SC, Chen WJ. Ultrasonographic screening of clinically-suspected necrotizing fasciitis. Acad Emerg Med. 2002;9(12):1448–51.
Becker M, Zbaren P, Hermans R, Becker CD, Marchal F, Kurt AM, et al. Necrotizing fasciitis of the head and neck: role of CT in diagnosis and management. Radiology. 1997;202(2):471–6.
Wysoki MG, Santora TA, Shah RM, Friedman AC. Necrotizing fasciitis: CT characteristics. Radiology. 1997;203(3):859–63.
Ma LD, Frassica FJ, Bluemke DA, Fishman EK. CT and MRI evaluation of musculoskeletal infection. Crit Rev Diagn Imaging. 1997;38(6):535–68.
Bloching M, Gudziol S, Gajda M, Berghaus A. Diagnosis and treatment of necrotizing fasciitis of the head and neck region. Laryngorhinootologie. 2000;79(12):774–9.
Lazow SK. Necrotizing fasciitis and mediastinitis. Atlas Oral Maxillofac Surg Clin North Am. 2000;8(1):101–19.
Arslan A, Pierre-Jerome C, Borthne A. Necrotizing fasciitis: unreliable MRI findings in the preoperative diagnosis. Emerg Radiol. 2000;36(3):139–43.
Yu JS, Habib P. MR imaging of urgent inflammatory and infectious conditions affecting the soft tissues of the musculoskeletal system. Emerg Radiol. 2009;16(4):267–76.
Schmid MR, Kossmann T, Duewell S. Differentiation of necrotizing fasciitis and cellulitis using MR imaging. AJR Am J Roentgenol. 1998;170(3):615–20.
Seok JH, Jee WH, Chun KA, Kim JY, Jung CK, Kim YR, et al. Necrotizing fasciitis versus pyomyositis: discrimination with using MR imaging. Korean J Radiol. 2009;10(2):121–8.
Kim KT, Kim YJ, Won Lee J, Kim YJ, Park SW, Lim MK, et al. Can necrotizing infectious fasciitis be differentiated from nonnecrotizing infectious fasciitis with MR imaging? Radiology. 2011;259(3):816–24.
Revelon G, Rahmouni A, Jazaerli N, Godeau B, Chosidow O, Authier J, et al. Acute swelling of the limbs: magnetic resonance pictorial review of fascial and muscle signal changes. Emerg Radiol. 1999;30(1):11–21.
Loh NN, Ch’en IY, Cheung LP, Li KC. Deep fascial hyperintensity in soft-tissue abnormalities as revealed by T2-weighted MR imaging. AJR Am J Roentgenol. 1997;168(5):1301–4.
Conflicts of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
We, the authors confirm that the article is not under consideration for publication elsewhere, each author has participated sufficiently in any submission to take public responsibility for its content, which has been approved by all authors and tacitly by the responsible authorities in our hospital in Valencia
Rights and permissions
About this article
Cite this article
Paz Maya, S., Dualde Beltrán, D., Lemercier, P. et al. Necrotizing fasciitis: an urgent diagnosis. Skeletal Radiol 43, 577–589 (2014). https://doi.org/10.1007/s00256-013-1813-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-013-1813-2