Abstract
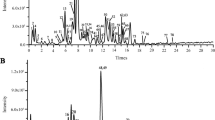
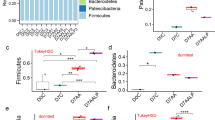
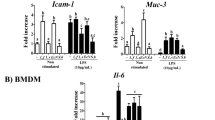
Baicalin is reported as an effective drug for ulcerative colitis (UC). However, its effect on gut microbiota and short-chain fatty acids (SCFAs) remains unknown. In this study, we investigated the role of baicalin on Th17/Treg balance, gut microbiota community, and SCFAs levels in trinitrobenzene sulphonic acid (TNBS)-induced UC rat model. We found the DAI scores were significantly increased in the TNBS-treated rats, while reduced in the baicalin-treated group in a dose-dependent manner, accompanied with the alleviation of mucosal injury, the reduction of ZO-1, Occludin, and MUC2 expression. At the meanwhile, baicalin repressed the increased levels of reactive oxygen species (ROS) and MDA, while deceased the GSH and SOD levels in colon tissue of rats treated with TNBS. On the other hand, administration of baicalin attenuated the TNBS-induced upregulations of Th17/Treg ratio, indicating a strong amelioration in the colorectal inflammation. More importantly, pyrosequencing of the V4 regions of 16S rRNA genes in rat feces revealed a deviation of the gut microbiota in response to baicalin treatment. In particular, the decreased Firmicutes-to-Bacteroidetes ratios and endotoxin-bearing Proteobacteria levels indicated that baicalin reversed TNBS-induced gut dysbiosis OTUs. In addition, we further investigated the fecal levels of major SCFAs in rats and found that baicalin significantly resorted the fecal butyrate levels in rats treated with TNBS. The increased butyrate levels were in consistent with the higher abundance of butyrate-producing species such as Butyricimonas spp., Roseburia spp., Subdoligranulum spp., and Eubacteriu spp. in baicalin-treated group. In conclusion, our findings suggest that baicalin possibly protected rats against ulcerative colitis by regulation of Th17/Treg balance, and modulation of both gut microbiota and SCFAs. Baicalin may be used as a prebiotic agent to treat ulcerative colitis-associated inflammation and gut dysbiosis.





Similar content being viewed by others
References
Barnich N, Darfeuille-Michaud A (2007) Role of bacteria in the etiopathogenesis of inflammatory bowel disease. World J Gastroenterol 13:5571–5576
Bellavia M, Tomasello G, Romeo M, Damiani P, Lo Monte AI, Lozio L, Campanella C, Marino Gammazza A, Rappa F, Zummo G, Cocchi M, Conway de Macario E, Macario AJ, Cappello F (2013) Gut microbiota imbalance and chaperoning system malfunction are central to ulcerative colitis pathogenesis and can be counteracted with specifically designed probiotics: a working hypothesis. Med Microbiol Immunol 202:393–406
Bettelli E, Oukka M, Kuchroo VK (2007) T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol 8:345–350
Chen GL, Zhang Y, Wang WY, Ji XL, Meng F, Xu PS, Yang NM, Ye FQ, Bo XC (2017) Partners of patients with ulcerative colitis exhibit a biologically relevant dysbiosis in fecal microbial metacommunities. World J Gastroenterol 23:4624–4631
Cooper HS, Murthy SN, Shah RS, Sedergran DJ (1993) Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Investig 69:238–249
Dai SX, Zou Y, Feng YL, Liu HB, Zheng XB (2012) Baicalin down-regulates the expression of macrophage migration inhibitory factor (MIF) effectively for rats with ulcerative colitis. Phytother Res 26:498–504
Eastaff-Leung N, Mabarrack N, Barbour A, Cummins A, Barry S (2010) Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J Clin Immunol 30:80–89
Harig JM, Soergel KH, Komorowski RA, Wood CM (1989) Treatment of diversion colitis with short-chain-fatty acid irrigation. N Engl J Med 320:23–28
Hori S, Nomura T, Sakaguchi S (2003) Control of regulatory T cell development by the transcription factor Foxp3. Science 299:1057–1061
Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC (2008) The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A 105:15064–15069
Kihara N, de la Fuente SG, Fujino K, Takahashi T, Pappas TN, Mantyh CR (2003) Vanilloid receptor-1 containing primary sensory neurones mediate dextran sulphate sodium induced colitis in rats. Gut 52:713–719
Li C, Zhao Y, Cheng J, Guo J, Zhang Q, Zhang X, Ren J, Wang F, Huang J, Hu H, Wang R, Zhang J (2019) A proresolving peptide nanotherapy for site-specific treatment of inflammatory bowel disease by regulating proinflammatory microenvironment and gut microbiota. Adv Sci (Weinh) 6(18):1900610
Lin M, Li L, Zhang Y, Zheng L, Xu M, Rong R, Zhu T (2014) Baicalin ameliorates H2O2 induced cytotoxicity in HK-2 cells through the inhibition of ER stress and the activation of Nrf2 signaling. Int J Mol Sci 15:12507–12522
Louis P, Flint HJ (2009) Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett 294:1–8
Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, Ballet V, Claes K, Van Immerseel F, Verbeke K, Ferrante M, Verhaegen J, Rutgeerts P, Vermeire S (2014) A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 63:1275–1283
Makhezer N, Ben Khemis M, Liu D, Khichane Y, Marzaioli V, Tlili A, Mojallali M, Pintard C, Letteron P, Hurtado-Nedelec M, El-Benna J, Marie JC, Sannier A, Pelletier AL, Dang PM (2019) NOX1-derived ROS drive the expression of Lipocalin-2 in colonic epithelial cells in inflammatory conditions. Mucosal Immunol 12(1):117–131
Moayyedi, P., Surette, M.G., Kim, P.T., Libertucci, J., Wolfe, M., Onischi, C., Armstrong, D., Marshall, J.K., Kassam, Z., Reinisch, W., and Lee, C.H. (2015). Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 149, 102–109 e106
Nemoto H, Kataoka K, Ishikawa H, Ikata K, Arimochi H, Iwasaki T, Ohnishi Y, Kuwahara T, Yasutomo K (2012) Reduced diversity and imbalance of fecal microbiota in patients with ulcerative colitis. Dig Dis Sci 57:2955–2964
Ni J, Wu GD, Albenberg L, Tomov VT (2017) Gut microbiota and IBD: causation or correlation? Nat Rev Gastroenterol Hepatol 14:573–584
Noor SO, Ridgway K, Scovell L, Kemsley EK, Lund EK, Jamieson C, Johnson IT, Narbad A (2010) Ulcerative colitis and irritable bowel patients exhibit distinct abnormalities of the gut microbiota. BMC Gastroenterol 10:134
Nusbaum DJ, Sun F, Ren J, Zhu Z, Ramsy N, Pervolarakis N, Kunde S, England W, Gao B, Fiehn O, Michail S, Whiteson K (2018) Gut microbial and metabolomic profiles after fecal microbiota transplantation in pediatric ulcerative colitis patients. FEMS Microbiol Ecol 94
Ordas I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ (2012) Ulcerative colitis. Lancet 380:1606–1619
Ott SJ, Musfeldt M, Wenderoth DF, Hampe J, Brant O, Folsch UR, Timmis KN, Schreiber S (2004) Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut 53:685–693
Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Li S, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K, Wang J (2012) A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490:55–60
Rossen NG, Fuentes S, van der Spek MJ, Tijssen JG, Hartman JH, Duflou A, Lowenberg M, van den Brink GR, Mathus-Vliegen EM, de Vos WM, Zoetendal EG, D'Haens GR, Ponsioen CY (2015) Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology 149(110–118):e114
Seksik P, Rigottier-Gois L, Gramet G, Sutren M, Pochart P, Marteau P, Jian R, Dore J (2003) Alterations of the dominant faecal bacterial groups in patients with Crohn’s disease of the colon. Gut 52:237–242
Sokol H, Seksik P, Rigottier-Gois L, Lay C, Lepage P, Podglajen I, Marteau P, Dore J (2006) Specificities of the fecal microbiota in inflammatory bowel disease. Inflamm Bowel Dis 12:106–111
Sun M, Wu W, Liu Z, Cong Y (2017) Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J Gastroenterol 52:1–8
Thompson GB, Pemberton JH, Morris S, Bustamante MA, Delong B, Carpenter HA, Wright AJ (1989) Kaposi's sarcoma of the colon in a young HIV-negative man with chronic ulcerative colitis. Report of a case. Dis Colon Rectum 32:73–76
Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN, Garrett WS (2013) The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341:569–573
Ueyama J, Nadai M, Kanazawa H, Iwase M, Nakayama H, Hashimoto K, Yokoi T, Baba K, Takagi K, Takagi K, Hasegawa T (2005) Endotoxin from various gram-negative bacteria has differential effects on function of hepatic cytochrome P450 and drug transporters. Eur J Pharmacol 510(1–2):127–134
van der Vliet A, Bast A (1992) Role of reactive oxygen species in intestinal diseases. Free Radic Biol Med 12:499–513
Wan J, Hu S, Ni K, Chang G, Sun X, Yu L (2016) Characterisation of fecal soap fatty acids, calcium contents, bacterial community and short-chain fatty acids in Sprague Dawley rats fed with different sn-2 Palmitic Triacylglycerols diets. PLoS One 11:e0164894
Yan ZX, Gao XJ, Li T, Wei B, Wang PP, Yang Y, Yan R (2018) Fecal microbiota transplantation in experimental ulcerative colitis reveals associated gut microbial and host metabolic reprogramming. Appl Environ Microbiol:84
Yu FY, Huang SG, Zhang HY, Ye H, Chi HG, Zou Y, Lv RX, Zheng XB (2014) Effects of Baicalin in CD4 + CD29 + T cell subsets of ulcerative colitis patients. World J Gastroenterol 20:15299–15309
Zhang CL, Zhang S, He WX, Lu JL, Xu YJ, Yang JY, Liu D (2017) Baicalin may alleviate inflammatory infiltration in dextran sodium sulfate-induced chronic ulcerative colitis via inhibiting IL-33 expression. Life Sci 186:125–132
Acknowledgments
This work was funded by the National Natural Science Foundation of China (No. 81873260 and No. 81873260), China Postdoctoral Science Foundation (No. 2018M640511), and the Special Scientific Research for Traditional Chinese Medicine of State Administration of Traditional Chinese Medicine of China (No. 201407001).
Availability of data and material
The datasets used during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
Lei Zhu and Hong Shen are major contributors in funding acquisition. Lei Zhu performed most of the experiments and date interpreting. Lu-Zhou Xu, Song Zhao, and Zhao-Feng Shen contributed in doing the experiments and date analysis. Hong Shen and Li-Bin Zhan were major contributors in project administration and manuscript writing. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
This study was approved by the Animal Care and Use Committee of Nanjing University of Chinese Medicine.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(XLSX 104 kb)
Rights and permissions
About this article
Cite this article
Zhu, L., Xu, LZ., Zhao, S. et al. Protective effect of baicalin on the regulation of Treg/Th17 balance, gut microbiota and short-chain fatty acids in rats with ulcerative colitis. Appl Microbiol Biotechnol 104, 5449–5460 (2020). https://doi.org/10.1007/s00253-020-10527-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10527-w