Abstract
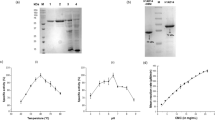
In a previous study, we reported an alkaliphilic and thermostable endoglucanase (BsGH7-3) of glycoside hydrolase family 7 (GH7) from the hemibiotrophic plant pathogen Bipolaris sorokiniana. However, the catalytic efficiency of the enzyme was lower than for some other endoglucanases of the GH7 family reported in the literature. To engineer a more active enzyme, we identified conserved residues in the substrate-binding tunnel and on the surface of the protein that could play a role in charge-charge interaction and stabilize the structure. The mutants D257W and Q225H in the substrate-binding tunnel and Y222R and Q401N on the protein surface showed a 2-fold increase in specific activity and a 1.5-fold increase in turnover number and were active over a broader range of pH. The mutants also showed a higher tolerance to NaCl. The rational design of the BsGH7-3 mutants helped in increasing the catalytic efficiency of the thermostable enzyme and may be useful in combination with other cellulases like cellobiohydrolase and β-glucosidase towards complete saccharification of cellulose into glucose.






Similar content being viewed by others
References
Aich S, Singh RK, Kundu P, Pandey SP, Datta S (2017) Genome-wide characterization of cellulases from the hemi-biotrophic plant pathogen, Bipolaris sorokiniana, reveals the presence of a highly stable GH7 endoglucanase. Biotechnol Biofuels 10(1):135
Argos P, Rossmann MG, Grau UM, Zuber H, Frank G, Tratschin JD (1979) Thermal stability and protein structure. Biochemistry 18(25):5698–5703
Aygan A, Arikan B (2008) A new halo-alkaliphilic, thermostable endoglucanase from moderately halophilic Bacillus sp. C14 isolated from van soda lake. Int J Agric Biol 10:369–374
Aygan A, Karcioglu L, Arikan B (2011) Alkaline thermostable and halophilic endoglucanase from Bacillus licheniformis C108. Afr J Biotechnol 10(5):789–796
Ben Hmad I, Boudabbous M, Belghith H, Gargouri A (2017) A novel ionic liquid-stable halophilic endoglucanase from Stachybotrys microspora. Process Biochem 54:59–66
Bharadwaj R, Chen Z, Datta S, Holmes BM, Sapra R, Simmons BA, Adams PD, Singh AK (2010) Microfluidic glycosyl hydrolase screening for biomass-to-biofuel conversion. Anal Chem 82(22):9513–9520
Borders CL Jr, Broadwater JA, Bekeny PA, Salmon JE, Lee AS, Eldridge AM, Pett VB (1994) A structural role for arginine in proteins: multiple hydrogen bonds to backbone carbonyl oxygens. Protein Sci 3(4):541–548
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brons-Poulsen J, Petersen NE, Horder M, Kristiansen K (1998) An improved PCR-based method for site directed mutagenesis using megaprimers. Mol Cell Probes 12(6):345–348
Creagh AL, Ong E, Jervis E, Kilburn DG, Haynes CA (1996) Binding of the cellulose-binding domain of exoglucanase Cex from Cellulomonas fimi to insoluble microcrystalline cellulose is entropically driven. Proc Natl Acad Sci U S A 93(22):12229–12234
Datta S (2016) Recent strategies to overexpress and engineer cellulases for biomass degradation. Curr Metabolomics 4(1):14–22
Datta S, Holmes B, Park JI, Chen Z, Dibble DC, Hadi M, Blanch HW, Simmons BA, Sapra R (2010) Ionic liquid tolerant hyperthermophilic cellulases for biomass pretreatment and hydrolysis. Green Chem 12(2):338–345
Dym O, Mevarech M, Sussman JL (1995) Structural features that stabilize halophilic malate dehydrogenase from an archaebacterium. Science 267(5202):1344–1346
Endo K, Hakamada Y, Takizawa S, Kubota H, Sumitomo N, Kobayashi T, Ito S (2001) A novel alkaline endoglucanase from an alkaliphilic Bacillus isolate: enzymatic properties, and nucleotide and deduced amino acid sequences. Appl Microbiol Biotechnol 57(1–2):109–116
Garg R, Srivastava R, Brahma V, Verma L, Karthikeyan S, Sahni G (2016) Biochemical and structural characterization of a novel halotolerant cellulase from soil metagenome. Sci Rep 6:39634
Goldstein MA, Takagi M, Hashida S, Shoseyov O, Doi RH, Segel IH (1993) Characterization of the cellulose-binding domain of the Clostridium cellulovorans cellulose-binding protein A. J Bacteriol 175(18):5762–5768
Graziano G, Merlino A (2014) Molecular bases of protein halotolerance. Biochim Biophys Acta 1844(4):850–858
Heinzelman P, Snow CD, Smith MA, Yu X, Kannan A, Boulware K, Villalobos A, Govindarajan S, Minshull J, Arnold FH (2009) SCHEMA recombination of a fungal cellulase uncovers a single mutation that contributes markedly to stability. J Biol Chem 284(39):26229–26233
Hirasawa K, Uchimura K, Kashiwa M, Grant WD, Ito S, Kobayashi T, Horikoshi K (2006) Salt-activated endoglucanase of a strain of alkaliphilic Bacillus agaradhaerens. Antonie Van Leeuwenhoek 89(2):211–219
Huang X, Shao Z, Hong Y, Lin L, Li C, Huang F, Wang H, Liu Z (2010) Cel8H, a novel endoglucanase from the halophilic bacterium Halomonas sp. S66-4: molecular cloning, heterogonous expression, and biochemical characterization. J Microbiol 48(3):318–324
Kern M, McGeehan JE, Streeter SD, Martin RNA, Besser K, Elias L, Eborall W, Malyon GP, Payne CM, Himmel ME, Schnorr K, Beckham GT, Cragg SM, Bruce NC, McQueen-Mason SJ (2013) Structural characterization of a unique marine animal family 7 cellobiohydrolase suggests a mechanism of cellulase salt tolerance. Proc Natl Acad Sci U S A 110(25):10189–10194
Li X, Yu HY (2013) Characterization of a halostable endoglucanase with organic solvent-tolerant property from Haloarcula sp. G10. Int J Biol Macromol 62:101–106
Liu Y, Dun B, Shi P, Ma R, Luo H, Bai Y, Xie X, Yao B (2015) A novel GH7 endo-β-1,4-glucanase from Neosartorya fischeri P1 with good thermostability, broad substrate specificity and potential application in the brewing industry. PLoS One 10(9):e0137485–e0137485
Loladze VV, Ibarra-Molero B, Sanchez-Ruiz JM, Makhatadze GI (1999) Engineering a thermostable protein via optimization of charge-charge interactions on the protein surface. Biochemistry 38(50):16419–16423
Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev 66(3):506–577
Madern D, Pfister C, Zaccai G (1995) Mutation at a single acidic amino acid enhances the halophilic behaviour of malate dehydrogenase from Haloarcula marismortui in physiological salts. Eur J Biochem 230(3):1088–1095
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
Payne CM, Knott BC, Mayes HB, Hansson H, Himmel ME, Sandgren M, Stahlberg J, Beckham GT (2015) Fungal cellulases. Chem Rev 115(3):1308–1448
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF chimera--a visualization system for exploratory research and analysis. J Comput Chem 25(13):1605–1612
Shi R, Li Z, Ye Q, Xu J, Liu Y (2013) Heterologous expression and characterization of a novel thermo-halotolerant endoglucanase Cel5H from Dictyoglomus thermophilum. Bioresour Technol 142:338–344
Spector S, Wang M, Carp SA, Robblee J, Hendsch ZS, Fairman R, Tidor B, Raleigh DP (2000) Rational modification of protein stability by the mutation of charged surface residues. Biochemistry 39(5):872–879
Strub C, Alies C, Lougarre A, Ladurantie C, Czaplicki J, Fournier D (2004) Mutation of exposed hydrophobic amino acids to arginine to increase protein stability. BMC Biochem 5:9
Vogt G, Argos P (1997) Protein thermal stability: hydrogen bonds or internal packing? Fold Des 2(4):S40–S546
Vogt G, Woell S, Argos P (1997) Protein thermal stability, hydrogen bonds, and ion pairs. J Mol Biol 269(4):631–643
Yeoman CJ, Han Y, Dodd D, Schroeder CM, Mackie RI, Cann IK (2010) Thermostable enzymes as biocatalysts in the biofuel industry. Adv Appl Microbiol 70:1–55
Zhang T, Datta S, Eichler J, Ivanova N, Axen SD, Kerfeld CA, Chen F, Kyrpides N, Hugenholtz P, Cheng J-F, Sale KL, Simmons B, Rubin E (2011) Identification of a haloalkaliphilic and thermostable cellulase with improved ionic liquid tolerance. Green Chem 13(8):2083–2090
Zhang L, Tang X, Cui D, Yao Z, Gao B, Jiang S, Yin B, Yuan YA, Wei D (2014) A method to rationally increase protein stability based on the charge-charge interaction, with application to lipase LipK107. Protein Sci 23(1):110–116
Zhao J, Shi P, Yuan T, Huang H, Li Z, Meng K, Yang P, Yao B (2012) Purification, gene cloning and characterization of an acidic beta-1,4-glucanase from Phialophora sp. G5 with potential applications in the brewing and feed industries. J Biosci Bioeng 114(4):379–384
Funding
This work was supported in part by the Science & Engineering Research Board (SERB), Government of India, EMR/2016/003705 (S.D.), and Academic Research Fund (IISER Kolkata). SA is supported by a Senior Research Fellowship from IISER Kolkata.
Author information
Authors and Affiliations
Contributions
SD and SA designed the study. SA performed the study. SD and SA analyzed the data. SD and SA wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 520 kb)
Rights and permissions
About this article
Cite this article
Aich, S., Datta, S. Engineering of a highly thermostable endoglucanase from the GH7 family of Bipolaris sorokiniana for higher catalytic efficiency. Appl Microbiol Biotechnol 104, 3935–3945 (2020). https://doi.org/10.1007/s00253-020-10515-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10515-0