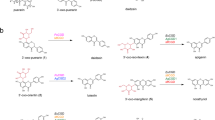
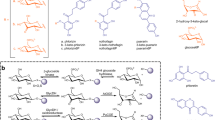
Abstract
C-Glycosides, a special type of glycoside, are frequently distributed in many kinds of medicinal plants, such as puerarin and mangiferin, showing various and significant bioactivities. C-Glycosides are usually characterized by the C–C bond that forms between the anomeric carbon of sugar moieties and the carbon atom of aglycon, which is usually resistant against acidic hydrolysis and enzymatic treatments. Interestingly, C-glycosides could be cleaved by several intestinal bacteria, but whether the enzymatic cleavage of C–C glycosidic bond is reduction or hydrolysis has been controversial; furthermore, whether existence of a “C-glycosidase” directly catalyzing the cleavage is not clear. Here we review research advances about the discovery and mechanism of intestinal bacteria in enzymatic cleavage of C–C glycosidic bond with an emphasis on the identification of enzymes manipulation the deglycosylation. Finally, we give a brief conclusion about the mechanism of C-glycoside deglycosylation and perspectives for future study in this field.


Similar content being viewed by others
References
Braune A, Blaut M (2011) Deglycosylation of puerarin and other aromatic C-glucosides by a newly isolated human intestinal bacterium. Environ Microbiol 13(2):482–494
Braune A, Blaut M (2012) Intestinal bacterium Eubacterium cellulosolvens deglycosylates flavonoid C- and O-glucosides. Appl Environ Microbiol 78(22):8151
Braune A, Engst W, Blaut M (2016) Identification and functional expression of genes encoding flavonoid O- and C-glycosidases in intestinal bacteria. Environ Microbiol 18(7):2117–2129
Che QM, Akao T, Hattori M, Kobashi K, Namba T (1991a) Isolation of a human intestinal bacterium capable of transforming barbaloin to aloe-emodin anthrone. Planta Med 57(01):15–19
Che QM, Akao T, Hattori M, Kobashi K, Namba T (1991b) Metabolism of aloesin and related compounds by human intestinal bacteria: a bacterial cleavage of the C-glucosyl bond and the subsequent reduction of the acetonyl side chain. Chem Pharm Bull 39(3):704–708
Che QM, Akao T, Hattori M, Tsuda Y, Namba T, Kobashi K (1991c) Barbaloin stimulates growth of Eubacterium sp. strain BAR, a barbaloin-metabolizing bacterium from human feces. Chem Pharm Bull 39(3):757–760
Hattori M, Kanda T, Shu YZ, Akao T, Kobashi K, Namba T (1988a) Metabolism of barbaloin by intestinal bacteria. Chem Pharm Bull 36(11):4462–4466
Hattori M, Shu YZ, El-Sedawy AI, Namba T, Kobashi K, Tomimori T (1988b) Metabolism of homoorientin by human intestinal bacteria. J Nat Prod 51(5):874
Hattori M, Shu YZ, Tomimori T, Kobashi K, Namba T (1989) A bacterial cleavage of the C-glucosyl bond of mangiferin and bergenin. Phytochemistry 28(4):1289–1290
Huang S, Mahanta N, Begley TP, Ealick SE (2012) Pseudouridine monophosphate glycosidase: a new glycosidase mechanism. Biochemistry 51(45):9245–9255
Ito T, Fujimoto S, Shimosaka M, Taguchi G (2014) Production of C-glucosides of flavonoids and related compounds by Escherichia coli expressing buckwheat C-glucosyltransferase. Plant Biotechnol 31(5):519–524
Jin JS, Nishihata T, Kakiuchi N, Hattori M (2008) Biotransformation of C-glucosylisoflavone puerarin to estrogenic (3S)-equol in co-culture of two human intestinal bacteria. Biol Pharm Bull 31(8):1621–1625
Kim DH, Jung EA, Sohng IS, Han JA, Kim TH, Han MJ (1998) Intestinal bacterial metabolism of flavonoids and its relation to some biological activities. Arch Pharm Res 21(1):17–23
Kim M, Lee J, Han J (2015) Deglycosylation of isoflavone C-glycosides by newly isolated human intestinal bacteria. J Sci Food Agric 95(9):1925–1931
Lairson LL, Henrissat B, Davies GJ, Withers SG (2008) Glycosyltransferases: structures, functions, and mechanisms. Annu Rev Biochem 77:521–555
Li Y, Meselhy MR, Wang LQ, Ma CM, Nakamura N, Hattori M (2000) Biotransformation of a C-glycosylflavone, abrusin 2-O-beta-D-apioside, by human intestinal bacteria. Chem Pharm Bull 48(8):1239–1241
Liu QP, Sulzenbacher G, Yuan HP, Bennett EP, Pietz G, Saunders K, Spence J, Nudelman E, Levery SB, White T (2007) Bacterial glycosidases for the production of universal red blood cells. Nat Biotechnol 25(4):454–464
Meselhy MR, Kadota S, Hattori M, Namba T (1993) Metabolism of safflor yellow B by human intestinal bacteria. J Nat Prod 56(1):39–45
Nakamura K, Nishihata T, Jin JS, Ma CM, Komatsu K, Iwashima M, Hattori M (2011) The C-glucosyl bond of puerarin was cleaved hydrolytically by a human intestinal bacterium strain PUE to yield its aglycone daidzein and an intact glucose. Chem Pharm Bull 59(1):23–27
Nakamura K, Komatsu K, Hattori M, Iwashima M (2013) Enzymatic cleavage of the C-glucosidic bond of puerarin by three proteins, Mn2+, and oxidized form of nicotinamide adenine dinucleotide. Biol Pharm Bull 36(4):635–640
Nakamura K, Zhu S, Komatsu K, Hattori M, Iwashima M (2019) Expression and characterization of the human intestinal bacterial enzyme which cleaves the C-glycosidic bond in 3-Oxo-puerarin. Biol Pharm Bull 42(3):417–423
Park EK, Shin J, Bae EA, Lee YC, Kim DH (2006) Intestinal bacteria activate estrogenic effect of main constituents puerarin and daidzin of pueraria thunbergiana. Biol Pharm Bull 29(12):2432–2435
Saito K (1990) Enzyme-catalyzed cleavage of the C-glycosidic linakage to the aromatic ring-a of a 3′,4′,5′7-tetrahydroxyflavone 8-C-glycoside. Biochim Biophys Acta 1035(3):340–347
Sanugul K, Akao T, Li Y, Kakiuchi N, Nakamura N, Hattori M (2005a) Isolation of a human intestinal bacterium that transforms mangiferin to norathyriol and inducibility of the enzyme that cleaves a C-glucosyl bond. Biol Pharm Bull 28(9):1672–1678
Sanugul K, Akao T, Nakamura N, Hattori M (2005b) Two proteins, Mn2+, and low molecular cofactor are required for C-glucosyl-cleavage of mangiferin. Biol Pharm Bull 28(11):2035
Van’t Slot G, Humpf HU (2009) Degradation and metabolism of catechin, epigallocatechin-3-gallate (EGCG), and related compounds by the intestinal microbiota in the pig cecum model. J Agric Food Chem 57(17):8041
Van’T Slot G, Mattern W, Rzeppa S, Grewe D, Humpf HU (2010) Complex flavonoids in cocoa: synthesis and degradation by intestinal microbiota. J Agric Food Chem 58(15):8879–8886
Wei B, Wang PP, Yan ZX, Yan R (2018) Characteristics and molecular determinants of a highly selective and efficient glycyrrhizin-hydrolyzing β-glucuronidase from Staphylococcus pasteuri 3I10. Appl Microbial Biot 102(21):9193–9205
Xiao J, Capanoglu E, Jassbi AR, Miron A (2016) Advance on the flavonoid C-glycosides and health benefits. Crit Rev Food Sci Nutr 56:S29–S45
Xu J, Qian D, Jiang S, Guo J, Shang EX, Duan JA, Yang J (2014) Application of ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry to determine the metabolites of orientin produced by human intestinal bacteria. J Chromatogr B Anal Technol Biomed Life Sci 944(3):123–127
Yasuda T, Kano Y, Saito K, Ohsawa K (1996) Urinary and biliary metabolites of puerarin in rats. Biol Pharm Bull 19(3):413
Yip VLY, Varrot A, Davies GJ, Rajan SS, Yang XJ, Thompson J, Anderson WF, Withers SG (2004) An unusual mechanism of glycoside hydrolysis involving redox and elimination steps by a family 4 beta-glycosidase from Thermotoga maritima. J Am Chem Soc 126(27):8354–8355
Zheng S, Geng D, Liu S, Wang Q, Liu S, Wang R (2019) A newly isolated human intestinal bacterium strain capable of deglycosylating flavone C-glycosides and its functional properties. Microb Cell Fact 18
Funding
This work was financially supported by the National Key R&D Program of China (2017YFE0103100), the National Natural Science Foundation of China (No. 81773628 and No. 81741165), and the National 111 Project (No. D17012).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wei, B., Wang, YK., Qiu, WH. et al. Discovery and mechanism of intestinal bacteria in enzymatic cleavage of C–C glycosidic bonds. Appl Microbiol Biotechnol 104, 1883–1890 (2020). https://doi.org/10.1007/s00253-019-10333-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-10333-z