Abstract
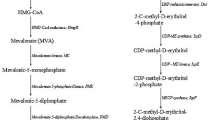
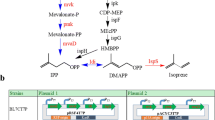
The biosynthesis of isoprene by microorganisms is a promising green route. However, the yield of isoprene is limited due to the generation of excess NAD(P)H via the mevalonate (MVA) pathway, which converts more glucose into CO2 or undesired reduced by-products. The production of 1,3-propanediol (1,3-PDO) from glycerol is a typical NAD(P)H-consuming process, which restricts 1,3-PDO yield to ~ 0.7 mol/mol. In this study, we propose a strategy of redox cofactor balance by coupling the production of isoprene with 1,3-PDO fermentation. With the introduction and optimization of the dual pathways in an engineered Escherichia coli, ~ 85.2% of the excess NADPH from isoprene pathway was recycled for 1,3-PDO production. The best strain G05 simultaneously produced 665.2 mg/L isoprene and 2532.1 mg/L 1,3-PDO under flask fermentation conditions. The yields were 0.3 mol/mol glucose and 1.0 mol/mol glycerol, respectively, showing 3.3- and 4.3-fold improvements relative to either pathway independently. Since isoprene is a volatile organic compound (VOC) whereas 1,3-PDO is separated from the fermentation broth, their coproduction process does not increase the complexity or cost for the separation from each other. Hence, the presented strategy will be especially useful for developing efficient biocatalysts for other biofuels and biochemicals, which are driven by cofactor concentrations.








Similar content being viewed by others
Availability of data and material
The data supporting the conclusions of this article are included within the article.
References
Ajikumar PK, Xiao WH, Tyo KE, Wang Y, Simeon F, Leonard E, Mucha O, Phon TH, Pfeifer B, Stephanopoulos G (2010) Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli. Science 330(6000):70–74
Beck ZQ, Cervin MA, Nielsen AT, Peres CM (2013) Compositions and methods of PGL for the increased production of isoprene. USA Patent US8455236B2
Biebl H, Menzel K, Zeng AP, Deckwer WD (1999) Microbial production of 1,3-propanediol. Appl Microbiol Biotechnol 52:289–297
Bohlmann J, Keeling CI (2008) Terpenoid biomaterials. Plant J 54(4):656–669
Cardenas J, Da Silva NA (2016) Engineering cofactor and transport mechanisms in Saccharomyces cerevisiae for enhanced acetyl-CoA and polyketide biosynthesis. Metab Eng 36:80–89
Carmona SB, Moreno F, Bolívar F, Gosset G, Escalante A (2015) Inactivation of the PTS as a strategy to engineer the production of aromatic metabolites in Escherichia coli. J Mol Microbiol Biotechnol 25:195–208
Celińska E (2010) Debottlenecking the 1,3-propanediol pathway by metabolic engineering. Biotechnol Adv 28(4):519–530
Chen X, Li S, Liu L (2014) Engineering redox balance through cofactor systems. Trends Biotechnol 32(6):337–343
Dhamankar H, Prather KL (2011) Microbial chemical factories: recent advances in pathway engineering for synthesis of value added chemicals. Curr Opin Struct Biol 21(4):488–494
Durnin G, Clomburg J, Yeates Z, Alvarez PJ, Zygourakis K, Campbell P, Gonzalez R (2009) Understanding and harnessing the microaerobic metabolism of glycerol in Escherichia coli. Biotechnol Bioeng 103(1):148–161
Edwards RA, Keller LH, Schifferli DM (1998) Improved allelic exchange vectors and their use to analyze 987P fimbria gene expression. Gene 207(2):149–157
Emptage M, Haynie SL, Laffend LA, Pucci JP, Whited G (2003) Process for the biological production of 1,3-propanediol with high titer. USA Patent US006514733B1
Feher FJ, Kan JK, Mcauliffe JC et al (2013) Purification of isoprene from renewable resources. USA patent 8569562 B2
Gao Y, Liu C, Ding Y, Sun C, Zhang R, Xian M, Zhao G (2014) Development of genetically stable Escherichia coli strains for poly(3-hydroxypropionate) production. PLoS One 9(5):e97845
Jarboe LR (2011) YqhD: a broad-substrate range aldehyde reductase with various applications in production of biorenewable fuels and chemicals. Appl Microbiol Biotechnol 89:249–257
Kaur G, Srivastava AK, Chand S (2012) Advances in biotechnological production of 1,3-propanediol. Biochem Eng J 64:106–118
Kim JH, Wang C, Jang HJ, Cha MS, Park JE, Jo SY, Choi ES, Kim SW (2016) Isoprene production by Escherichia coli through the exogenous mevalonate pathway with reduced formation of fermentation byproducts. Microb Cell Factories 15(1):214
King ZA, Feist AM (2014) Optimal cofactor swapping can increase the theoretical yield for chemical production in Escherichia coli and Saccharomyces cerevisiae. Metab Eng 24:117–128
Lemuth K, Steuer K, Albermann C (2011) Engineering of a plasmid-free Escherichia coli strain for improved in vivo biosynthesis of astaxanthin. Microb Cell Factories 10:29
Lindberg P, Park S, Melis A (2010) Engineering a platform for photosynthetic isoprene production in cyanobacteria, using Synechocystis as the model organism. Metab Eng 12(1):70–79
Liu M, Tolstorukov M, Zhurkin V, Garges S, Adhya S (2004) A mutant spacer sequence between −35 and −10 elements makes the P lac promoter hyperactive and cAMP receptor protein-independent. Proc Natl Acad Sci U S A 101(18):6911–6916
Lombó F, Pfeifer B, Leaf T, Ou S, Kim YS, Cane DE, Licari P, Khosla C (2001) Enhancing the atom economy of polyketide biosynthetic processes through metabolic engineering. Biotechnol Prog 17(4):612–617
Lv X, Xie W, Lu W, Guo F, Gu J, Yu H, Ye L (2014) Enhanced isoprene biosynthesis in Saccharomyces cerevisiae by engineering of the native acetyl-coA and mevalonic acid pathways with a push-pull-restrain strategy. J Biotechnol 186:128–136
Ma Z, Shentu X, Bian Y, Yu X (2013) Effects of NADH availability on the Klebsiella pneumoniae strain with 1,3-propanediol operon over-expression. J Basic Microbiol 53(4):348–354
Nakamura CE, Whited GM (2003) Metabolic engineering for the microbial production of 1,3-propanediol. Curr Opin Biotechnol 14(5):454–459
Roland K, Curtiss R 3rd, Sizemore D (1999) Construction and evaluation of a delta cya delta crp Salmonella typhimurium strain expressing avian pathogenic Escherichia coli O78 LPS as a vaccine to prevent airsacculitis in chickens. Avian Dis 43(3):429–441
Sauer U, Canonaco F, Heri S, Perrenoud A, Fischer E (2004) The soluble and membrane-bound transhydrogenases UdhA and PntAB have divergent functions in NADPH metabolism of Escherichia coli. J Biol Chem 279(8):6613–6619
Saxena RK, Anand P, Saran S, Isar J (2009) Microbial production of 1,3-propanediol: recent developments and emerging opportunities. Biotechnol Adv 27(6):895–913
Sulzenbacher G, Alvarez K, Van Den Heuvel RH, Versluis C, Spinelli S, Campanacci V, Valencia C, Cambillau C, Eklund H, Tegoni M (2004) Crystal structure of E. coli alcohol dehydrogenase YqhD: evidence of a covalently modified NADP coenzyme. J Mol Biol 342(2):489–502
Trost BM (1991) The atom economy—a search for synthetic efficiency. Science 254(5037):1471–1477
Vickers CE, Sabri S (2015) Isoprene. Adv Biochem Eng Biotechnol 148:289–317
Wada K, Toya Y, Banno S, Yoshikawa K, Matsuda F, Shimizu H (2017) 13C-metabolic flux analysis for mevalonate-producing strain of Escherichia coli. J Biosci Bioeng 123(2):177–182
Wang Y, San KY, Bennett GN (2013) Cofactor engineering for advancing chemical biotechnology. Curr Opin Biotechnol 24(6):994–999
Wang C, Pfleger BF, Kim SW (2017) Reassessing Escherichia coli as a cell factory for biofuel production. Curr Opin Biotechnol 45:92–103
Wei Y, Yuan C, Li J, Xu S, Zhou Y, Chen J, Wang Q, Xu L, Qi Y, Zhang Q, Liu Z (2012) Coke formation and carbon atom economy of methanol-to-olefins reaction. ChemSusChem 5(5):906–912
Whited GM, Feher FJ, Benko DA, Cervin MA, Chotani GK, McAuliffe JC, LaDuca RJ, BenShoshan EA, Sanford KJ (2010) Development of a gas-phase bioprocess for isoprene monomer production using metabolic pathway engineering. Ind Biotechnol 6(3):152–163
Xue J, Balamurugan S, Li DW, Liu YH, Zeng H, Wang L, Yang WD, Liu JS, Li HY (2017) Glucose-6-phosphate dehydrogenase as a target for highly efficient fatty acid biosynthesis in microalgae by enhancing NADPH supply. Metab Eng 41:212–221
Yang J, Xian M, Su S, Zhao G, Nie Q, Jiang X, Zheng Y, Liu W (2012a) Enhancing production of bio-isoprene using hybrid MVA pathway and isoprene synthase in E. coli. PLoS One 7(4):e33509
Yang J, Zhao G, Sun Y, Zheng Y, Jiang X, Liu W, Xian M (2012b) Bio-isoprene production using exogenous MVA pathway and isoprene synthase in Escherichia coli. Bioresour Technol 104:642–647
Yang C, Gao X, Jiang Y, Sun B, Gao F, Yang S (2016) Synergy between methylerythritol phosphate pathway and mevalonate pathway for isoprene production in Escherichia coli. Metab Eng 37:79–91
Ye L, Lv X, Yu H (2016) Engineering microbes for isoprene production. Metab Eng 38:125–138
Zeng AP, Biebl H (2002) Bulk chemicals from biotechnology: the case of 1,3-propanediol production and the new trends. Adv Biochem Eng Biotechnol 74:239–259
Zhuge B, Zhang C, Fang H, Zhuge J, Permaul K (2010) Expression of 1,3-propanediol oxidoreductase and its isoenzyme in Klebsiella pneumoniae for bioconversion of glycerol into 1,3-propanediol. Appl Microbiol Biotechnol 87(6):2177–2184
Acknowledgements
The authors appreciate the contribution of the Dr. Haibo Zhang for his advice on strain design. The authors also acknowledge Prof. Haiyan Yang for the assistance on the conduction of GC and HPLC detection.
Funding
This work was supported by Natural Science Foundation of China (No. 21572242), and Taishan Scholars Climbing Program of Shandong (No. tspd20150210).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 168 kb)
Rights and permissions
About this article
Cite this article
Guo, J., Cao, Y., Liu, H. et al. Improving the production of isoprene and 1,3-propanediol by metabolically engineered Escherichia coli through recycling redox cofactor between the dual pathways. Appl Microbiol Biotechnol 103, 2597–2608 (2019). https://doi.org/10.1007/s00253-018-09578-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-09578-x