Abstract
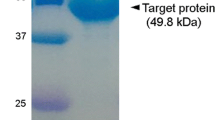
β-1,6-glucan is a polysaccharide found in brown macroalgae and fungal cell walls. In this study, a β-1,6-endoglucanase gene from Saccharophagus degradans 2-40T, gly30B, was cloned and overexpressed in Escherichia coli. Gly30B, which belongs to the glycoside hydrolase family 30 (GH30), was found to possess β-1,6-endoglucanase activity by hydrolyzing β-1,6-glycosidic linkages of pustulan (β-1,6-glucan derived from fungal cell walls) and laminarin (β-1,3-glucan with β-1,6-branchings, derived from brown macroalgae) to produce gentiobiose and glucose as the final products. The optimal pH and temperature for Gly30B activity were found to be pH 7.0 and 40 °C, respectively. The kinetic constants of Gly30B, V max, K M, and k cat were determined to be 153.8 U/mg protein, 24.2 g/L, and 135.6 s−1 for pustulan and 32.8 U/mg protein, 100.8 g/L, and 28.9 s−1 for laminarin, respectively. To our knowledge, Gly30B is the first β-1,6-endoglucanase characterized from bacteria. Gly30B can be used to hydrolyze β-1,6-glucans of brown algae or fungal cell walls for producing gentiobiose as a high-value sugar and glucose as a fermentable sugar.






Similar content being viewed by others
References
Boisramé A, Gaillardin C (2009) Heterologous expression and characterization of a β-1,6-glucanase from Aspergillus fumigatus. Appl Microbiol Biotechnol 82:663–669
Bryant MK, May KJ, Bryan GT, Scott B (2007) Functional analysis of a β-1,6-glucanase gene from the grass endophytic fungus Epichloë festucae. Fungal Genet Biol 44:808–817
Cruz J, Pintor-Toro JA, Benitez T, Llobell A (1995) Purification and characterization of an endo-β-1,6-glucanase from Trichoderma harzianum that is related to its mycoparasitism. J Bacteriol 177:1864–1871
Djonović S, Pozo MJ, Kenerley CM (2006) Tvbgn3, a β-1,6-glucanase from the biocontrol fungus Trichoderma virens, is involved in mycoparasitism and control of Pythium ultimum. Appl Environ Microbiol 72:7661–7670
Ekborg NA, Gonzalez JM, Howard MB, Taylor LE, Hutcheson SW, Weiner RM (2005) Saccharophagus degradans gen. nov., sp. nov., a versatile marine degrader of complex polysaccharides. Int J Syst Evol Microbiol 55:1545–1549
Ensor L, Stosz SK, Weiner RM (1999) Expression of multiple insoluble complex polysaccharide degrading enzyme systems by a marine bacterium strain 2-40. J Ind Microbiol Biotechnol 23:123–126
Gibson SR, Roberfroid MB (1995) Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr 125:1401–1412
Ha SC, Lee S, Lee J, Kim HT, Ko H-J, Kim KH, Choi I-G (2011) Crystal structure of a key enzyme in the agarolytic pathway, α-neoagarobiose hydrolase from Saccharophagus degradans 2-40. Biochem Biophys Res Commun 412:238–244
Howard MB, Ekborg NA, Taylor LE, Weiner RM, Hutcheson SW (2003) Genomic analysis and initial characterization of the chitinolytic system of Microbulbifer degradans strain 2-40. J Bacteriol 185:3352–3360
Hutcheson SW, Zhang H, Suvorov M (2011) Carbohydrase systems of Saccharophagus degradans degrading marine complex polysaccharides. Mar Drugs 9:645–665
Kawai R, Igarashi K, Kitaoka M, Ishii T, Samejima M (2004) Kinetics of substrate transglycosylation by glycoside hydrolase family 3 glucan (1→3)-β-glucosidease from the white-rot fungus Phanerochaete chrysosporium. Carbohydr Res 339:2851–2857
Kim HT, Lee S, Lee D, Kim H-S, Bang W-G, Kim KH, Choi I-G (2010) Overexpression and molecular characterization of Aga50D from Saccharophagus degradans 2-40: an exo-type β-agarase producing neoagarobiose. Appl Microbiol Biotechnol 86:227–234
Kim HT, Chung JH, Wang D, Lee J, Woo HC, Choi I-G, Kim KH (2012) Depolymerization of alginate into a monomeric sugar acid using Alg17C, an exo-oligoalginate lyase cloned from Saccharophagus degradans 2-40. Appl Microbiol Biotechnol 93:2233–2239
Klis FM (1994) Review: cell wall assembly in yeast. Yeast 10:851–869
Ko JK, Jung MW, Kim KH, Choi I-G (2009) Optimal production of a novel endo-acting β-1,4-xylanase cloned from Saccharophagus degradans 2-40 into Escherichia coli BL21(DE3). New Biotechnol 26:157–164
Kollár R, Reinhold BB, Petráková E, Yeh HJ, Ashwell G, Drgonová J, Kapteyn JC, Klis FM, Cabib E (1997) Architecture of the yeast cell wall: β(1→6)-glucan interconnects mannoprotein, β(1→3)-glucan, and chitin. J Biol Chem 272:17762–17775
Lora JM, De la Cruz J, Llobell A, Benítez T, Pintor-Toro JA (1995) Molecular characterization and heterologous expression of an endo-β-1,6 glucanase gene from the mycoparasitic fungus Trichoderma harzianum. Mol Gen Genet 247:639–645
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Montero M, Sanz L, Rey M, Llobell A, Monte E (2007) Cloning and characterization of bgn16.3, coding for a β-1,6-glucanase expressed during Trichoderma harzianum mycoparasitism. J Appl Microbiol 103:1291–1300
Nishikawa Y, Tanaka M, Shibata S, Fukuoka F (1970) Polysaccharides of lichens and fungi. IV Antitumour active O-acetylated pustulan-type glucans from the lichens of Umbilicaria species. Chem Pharm Bull 18:1431–1434
Rombouts FM, Phaff HJ (1976) Lysis of yeast cell walls. Lytic β-1,6-glucanase from Bacillus circulans WL-12. Eur J Biochem 63:109–120
Ruiz-Herrera J, Elorza MV, Valentín E, Sentandreu R (2006) Molecular organization of the cell wall of Candida albicans and its relation to pathogenicity. FEMS Yeast Res 6:14–29
Sakaguchi K, Okino N, Izu H, Ito M (1999) The Glu residue in the conserved Asn-Glu-Pro sequence of endoglycoceramidase is essential for enzymatic activity. Biochem Biophys Res Commun 260:89–93
Shin HJ, Oh SJ, Kim SI, Kim HW, Son J-H (2009) Conformational characteristics of β-glucan in laminarin probed by terahertz spectroscopy. Appl Phys Lett 94:1119111–1119113
St John FJ, Gonzalez JM, Pozharski E (2010) Consolidation of glycosyl hydrolase family 30: a dual domain 4/7 hydrolase family consisting of two structurally distinct groups. FEBS Lett 584:4435–4441
Takeshi H, Yasuna K, Shuji U, Taichi U (2013) Mode of action of a β-(1→6)-glucanase from Penicillium multicolor. Carbohydr Res 366:6–16
Taylor LE, Henrissat B, Coutinho PM, Ekborg NA, Hutcheson SW, Weiner RM (2006) Complete cellulase system in the marine bacterium Saccharophagus degradans strain 2-40T. J Bacteriol 188:3849–3861
Yamamoto S, Kobayashi R, Nagasaki S (1974) Purification and properties of an endo β-1,6-glucanase from Rhizopus chinensis R-69. Agric Biol Chem 38:1493–1500
Acknowledgements
This work was financially supported by the Advanced Biomass R&D Center of Korea (2011-0031359), funded by the Korean Government (MSIP). Experiments were carried out using the facilities of the Institute of Biomedical Science and Food Safety at the Food Safety Hall, Korea University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, D., Kim, D.H., Yun, E.J. et al. The first bacterial β-1,6-endoglucanase from Saccharophagus degradans 2-40T for the hydrolysis of pustulan and laminarin. Appl Microbiol Biotechnol 101, 197–204 (2017). https://doi.org/10.1007/s00253-016-7753-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7753-8