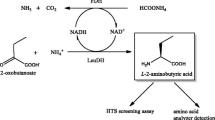
Abstract
l-tert-Leucine and its derivatives are used as synthetic building blocks for pharmaceutical active ingredients, chiral auxiliaries, and ligands. Leucine dehydrogenase (LeuDH) is frequently used to prepare l-tert-leucine from the α-keto acid precursor trimethylpyruvate (TMP). In this study, a high-throughput screening method for the l-tert-leucine synthesis reaction based on a spectrophotometric approach was developed. Directed evolution strategy was applied to engineer LeuDH from Lysinibacillus sphaericus for improved efficiency of l-tert-leucine synthesis. After two rounds of random mutagenesis, the specific activity of LeuDH on the substrate TMP was enhanced by more than two-fold, compared with that of the wild-type enzyme, while the activity towards its natural substrate, leucine, decreased. The catalytic efficiencies (k cat/K m) of the best mutant enzyme, H6, on substrates TMP and NADH were all enhanced by more than five-fold as compared with that of the wild-type enzyme. The efficiency of l-tert-leucine synthesis by mutant H6 was significantly improved. A productivity of 1170 g/l/day was achieved for the mutant enzyme H6, compared with 666 g/l/day for the wild-type enzyme.






Similar content being viewed by others
References
Ansorge-Schumacher MB, Slusarczyk H, Schumers J, Hirtz D (2006) Directed evolution of formate dehydrogenase from Candida boidinii for improved stability during entrapment in polyacrylamide. FEBS J 273:3938–3945
Ariga N (1972) Thin-layer chromatography of keto acid 2,4-dinitrophenylhydrazones. Anal Biochem 49:436–441
Baik SH, Ide T, Yoshida H, Kagami O, Harayama S (2003) Significantly enhanced stability of glucose dehydrogenase by directed evolution. Appl Microbiol Biotechnol 61:329–335
Baker PJ, Turnbull AP, Sedelnikova SE, Stillman TJ, Rice DW (1995) A role for quaternary structure in the substrate-specificity of leucine dehydrogenase. Structure 3:693–705
Bhushan R, Bruckner H (2004) Marfey’s reagent for chiral amino acid analysis: a review. Amino Acids 27:231–247
Carter JLL, Bekhouche M, Noiriel A, Blum LJ, Doumeche B (2014) Directed evolution of a formate dehydrogenase for increased tolerance to ionic liquids reveals a new site for increasing the stability. Chembiochem 15:2710–2718
Chen S, Engel PC (2009) Efficient screening for new amino acid dehydrogenase activity: directed evolution of Bacillus sphaericus phenylalanine dehydrogenase towards activity with an unsaturated non-natural amino acid. J Biotechnol 142:127–134
Dietrich JA, McKee AE, Keasling JD (2010) High-throughput metabolic engineering: advances in small-molecule screening and selection. Annu Rev Biochem 79:563–590
Fassler A, Bold G, Capraro H-G, Lang M, Khanna SC (1997) Antivirally active heterocyclic azahexane derivatives. US patent US5849911 A.
He YC, Zhang DP, Tao ZC, Lu Y, Ding Y, Liu F, Zhu ZZ, Rui H, Zheng GW, Zhang X (2015) Improved biosynthesis of ethyl (S)-4-chloro-3-hydroxybutanoate by adding L-glutamine plus glycine instead of NAD in beta-cyclodextrinwater system. Bioresour Technol 182:98–102
Hess S, Gustafson KR, Milanowski DJ, Alvira E, Lipton MA, Pannell LK (2004) Chirality determination of unusual amino acids using precolumn derivatization and liquid chromatography-electrospray ionization mass spectrometry. J Chromatogr A 1035:211–219
Hong EY, Cha MH, Yun HD, Kim BG (2010) Asymmetric synthesis of l-tert-leucine and l-3-hydroxyadamantylglycine using branched chain aminotransferase. J MolCatal B Enzym 66:228–233
Hupert-Kocurek K, Banas A, Wojcieszynska D, Guzik U (2014) Directed evolution of microbial enzymes. Postepy Mikrobiologii 53:43–48
Imamura C, Shigemori Y (2010) Enhancement of thermal stabilization of formaldehyde dehydrogenase from Pseudomonas putida by directed evolution. Biosci Biotechnol Biochem 74:1462–1465
Johannes TW, Woodyer RD, Zhao H (2005) Directed evolution of a thermostable phosphite dehydrogenase for NAD(P)H regeneration. Appl Environ Microbiol 71:5728–5734
Joshi S, Satyanarayana T (2015) In vitro engineering of microbial enzymes with multifarious applications: prospects and perspectives. Bioresour Technol 176:273–283
Kataoka K, Tanizawa K (2003) Alteration of substrate specificity of leucine dehydrogenase by site-directed mutagenesis. J Mol Catal B Enzym 23:299–309
Kim JY, Lee YA, Wittmann C, Park JB (2013) Production of non-proteinogenic amino acids from α-keto acid precursors with recombinant Corynebacterium glutamicum. Biotechnol Bioeng 110:2846–2855
Kragl U, Kruse W, Hummel W, Wandrey C (1996a) Enzyme engineering aspects of biocatalysis: cofactor regeneration as example. Biotechnol Bioeng 52:309–319
Kragl U, VasicRacki D, Wandrey C (1996b) Continuous production of l-tert-leucine in series of two enzyme membrane reactors—modelling and computer simulation. Bioprocess Eng 14:291–297
Laumen K, Kittelmann M, Ghisalba O (2002) Chemo-enzymatic approaches for the creation of novel chiral building blocks and reagents for pharmaceutical applications. J Mol Catal B Enzym 19:55–66
Li J, Pan J, Zhang J, Xu JH (2014) Stereoselective synthesis of l-tert-leucine by a newly cloned leucine dehydrogenase from Exiguobacterium sibiricum. J Mol Catal B Enzym 105:11–17
Liu WM, Ma HM, Luo JX, Shen WH, Xu X, Li S, Hu Y, Huang H (2014) Efficient synthesis of l-tert-leucine through reductive amination using leucine dehydrogenase and formate dehydrogenase coexpressed in recombinant E. coli. Biochem Eng J 91:204–209
Menzel A, Werner H, Altenbuchner J, Groger H (2004) From enzymes to “designer bugs” in reductive amination: a new process for the synthesis of l-tert-leucine using a whole cell-catalyst. Eng Life Sci 4:573–576
Oyobiki R, Kato T, Katayama M, Sugitani A, Watanabe T, Einaga Y, Matsumoto Y, Horisawa K, Doi N (2014) Toward high-throughput screening of NAD(P)-dependent oxidoreductases using boron-doped diamond microelectrodes and microfluidic devices. Anal Chem 86:9570–9575
Phillips GJ, Park SK, Huber D (2000) High copy number plasmids compatible with commonly used cloning vectors. BioTechniques 28:400–402, 404, 406 passim.
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28:56–63
Seo YM, Yun H (2011) Enzymatic synthesis of l-tert-leucine with branched chain aminotransferase. J Microbiol Biotechnol 21:1049–1052
Solano DM, Hoyos P, Hernaiz MJ, Alcantara AR, Sanchez-Montero JM (2012) Industrial biotransformations in the synthesis of building blocks leading to enantiopure drugs. Bioresour Technol 115:196–207
Steffler F, Guterl JK, Sieber V (2013) Improvement of thermostable aldehyde dehydrogenase by directed evolution for application in synthetic cascade biomanufacturing. Enzym Microb Technol 53:307–314
Tracewell CA, Arnold FH (2009) Directed enzyme evolution: climbing fitness peaks one amino acid at a time. Curr Opin Chem Biol 13:3–9
Weinhandl K, Winkler M, Glieder A, Camattari A (2012) A novel multi-enzymatic high throughput assay for transaminase activity. Tetrahedron 68:7586–7590
Xu ZM, Singh J, Schwinden MD, Zheng B, Kissick TP, Patel B, Humora MJ, Quiroz F, Dong L, Hsieh DM, Heikes JE, Pudipeddi M, Lindrud MD, Srivastava SK, Kronenthal DR, Mueller RH (2002) Process research and development for an efficient synthesis of the HIV protease inhibitor BMS-232632. Org Process Res Dev 6:323–328
Zhao HM (2007) Directed evolution of novel protein functions. Biotechnol Bioeng 98:313–317
Acknowledgments
This work was supported by the Ministry of Science and Technology of China Grant 2013CB734003 and the National Natural Science Foundation of China (Grant No. 21172095, 31160017, 21472234). We are grateful to Dr. Rongsheng Tao from Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, for providing us gene gdh from B. subtilis 168.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Lin Zhu and Zhe Wu contributed equally to this work.
Electronic Supplementary Material
ESM 1
(PDF 676 kb)
Rights and permissions
About this article
Cite this article
Zhu, L., Wu, Z., Jin, JM. et al. Directed evolution of leucine dehydrogenase for improved efficiency of l-tert-leucine synthesis. Appl Microbiol Biotechnol 100, 5805–5813 (2016). https://doi.org/10.1007/s00253-016-7371-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7371-5