Abstract
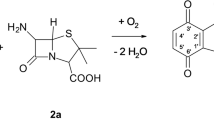
The rapidly increasing problem of antimicrobial-drug resistance requires the development of new antimicrobial agents. The laccase-catalyzed amination of dihydroxy aromatics is a new and promising method to enlarge the range of currently available antibiotics. Thirty-eight potential 1,2- and 1,4-hydroquinoid laccase substrates were screened for their antibacterial and cytotoxic activity to select the best substrates for laccase-catalyzed coupling reaction resulting in potent antibacterial derivatives. As a result, methyl-1,4-hydroquinone and 2,3-dimethyl-1,4-hydroquinone were used as parent compounds and 14 novel cephalosporins, penicillins, and carbacephems were synthesized by amination with amino-β-lactam structures. All purified products were stable in aqueous buffer and resistant to the action of β-lactamases, and in agar diffusion and broth micro-dilution assays, they inhibited the growth of several Gram-positive bacterial strains including multidrug-resistant Staphylococcus aureus and Enterococci. Their in vivo activity and cytotoxicity in a Staphylococcus-infected, immune-suppressed mouse model are discussed.





Similar content being viewed by others
References
Appelbaum PC (1987) World-wide development of antibiotic resistance in pneumococci. Eur J Clin Microbiol 6(4):367–377. doi:10.1007/bf02013089
Burkhardt F (1992) Mikrobiologische Diagnostik. Georg Thieme Verlag, Stuttgart
Ciecholewski S, Hammer E, Manda K, Bose G, Nguyen VTH, Langer P, Schauer F (2005) Laccase-catalyzed carbon-carbon bond formation: oxidative dimerization of salicylic esters by air in aqueous solution. Tetrahedron 61(19):4615–4619. doi:10.1016/j.tet.2005.03.007
Critchley IA, Karlowsky JA, Draghi DC, Jones ME, Thornsberry C, Murfitt K, Sahm DF (2002) Activities of faropenem, an oral β-lactam, against recent US isolates of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Antimicrob Agents Chemother 46(2):550–555. doi:10.1128/aac.46.2.550-555.2002
Fisher JF, Meroueh SO, Mobashery S (2005) Bacterial resistance to β-lactam antibiotics: compelling opportunism, compelling opportunity. Chem Rev 105(2):395–424. doi:10.1021/cr030102i
Fluit AC, Schmitz FJ, Jones ME, Acar J, Gupta R, Verhoef J (1999) Antimicrobial resistance among community-acquired pneumonia isolates in Europe: first results from the SENTRY antimicrobial surveillance program 1997. SENTRY Participants Group. Int J Infect Dis 3(3):153–156. doi:10.1016/s1201-9712(99)90037-1
Hahn V, Mikolasch A, Manda K, Goerdes D, Thurow K, Schauer F (2009) Laccase-catalyzed carbon-nitrogen bond formation: coupling and derivatization of unprotected l-phenylalanine with different para-hydroquinones. Amino Acids 37(2):315–321. doi:10.1007/s00726-008-0154-2
Heinzkill M, Bech L, Halkier T, Schneider P, Anke T (1998) Characterization of laccases and peroxidases from wood-rotting fungi (family Coprinaceae). Appl Environ Microbiol 64(5):1601–1606
Helfand MS, Bonomo RA (2005) Current challenges in antimicrobial chemotherapy: the impact of extended-spectrum β-lactamases and metallo-β-lactamases on the treatment of resistant gram-negative pathogens. Curr Opin Pharmacol 5(5):452–458. doi:10.1016/j.coph.2005.04.013
Huttner A, Harbarth S, Carlet J, Cosgrove S, Goossens H, Holmes A, Jarlier V, Voss A, Pittet D (2013) Antimicrobial resistance: a global view from the 2013 World Healthcare-Associated Infections Forum. Antimicrob Resist Infect Control 2:31. doi:10.1186/2047-2994-2-31
Intra A, Nicotra S, Riva S, Danieli B (2005) Significant and unexpected solvent influence on the selectivity of laccase-catalyzed coupling of tetrahydro-2-naphthol derivatives. Adv Synth Catal 347(7-8):973–977. doi:10.1002/adsc.200505043
Klugman KP (1990) Pneumococcal resistance to antibiotics. Clin Microbiol Rev 3(2):171–196
Koeck R, Becker K, Cookson B, van Gemert-Pijnen JE, Harbarth S, Kluytmans J, Mielke M, Peters G, Skov RL, Struelens MJ, Tacconelli E, Torne AN, Witte W, Friedrich AW (2010) Methicillin-resistant Staphylococcus aureus (MRSA): burden of disease and control challenges in Europe. Euro Surveill 15(41):12–20
Lindl T, Bauer J (1989) Zell und Gewebekultur. Gustav Fischer Verlag, Jena
Macal CM, North MJ, Collier N, Dukic VM, Wegener DT, David MZ, Daum RS, Schumm P, Evans JA, Wilder JR, Miller LG, Eells SJ, Lauderdale DS (2014) Modeling the transmission of community-associated methicillin-resistant Staphylococcus aureus: a dynamic agent-based simulation. J Transl Med 12:124–136. doi:10.1186/1479-5876-12-124
Manda K, Hammer E, Mikolasch A, Niedermeyer T, Dec J, Jones AD, Benesi AJ, Schauer F, Bollag JM (2005) Laccase-induced cross-coupling of 4-aminobenzoic acid with para-dihydroxylated compounds 2,5-dihydroxy-N-(2-hydroxyethyl)-benzamide and 2,5-dihydroxybenzoic acid methyl ester. J Mol Catal B-Enzym 35(4-6):86–92. doi:10.1016/j.molcatb.2005.06.001
Manda K, Hammer E, Mikolasch A, Gordes D, Thurow K, Schauer F (2006) Laccase-induced derivatization of unprotected amino acid L-tryptophan by coupling with p-hydroquinone 2,5-dihydroxy-N-(2-hydroxyethyl)-benzamide. Amino Acids 31(4):409–419
Manda K, Goerdes D, Mikolasch A, Hammer E, Schmidt E, Thurow K, Schauer F (2007) Carbon-oxygen bond formation by fungal laccases: cross-coupling of 2,5-dihydroxy-N-(2-hydroxyethyl)-benzamide with the solvents water, methanol, and other alcohols. Appl Microbiol Biotechnol 76(2):407–416. doi:10.1007/s00253-007-1024-7
Mann CM, Markham JL (1998) A new method for determining the minimum inhibitory concentration of essential oils. J Appl Microbiol 84(4):538–544. doi:10.1046/j.1365-2672.1998.00379.x
Mikolasch A, Niedermeyer T, Lalk M, Witt S, Seefeldt S, Hammer E, Schauer F, Gesell M, Hessel S, Juelich WD, Lindequist U (2006) Novel penicillins synthesized by biotransformation using laccase from Trametes spec. Chem Pharm Bull 54(5):632–638. doi:10.1248/cpb.54.632
Mikolasch A, Niedermeyer T, Lalk M, Witt S, Seefeldt S, Hammer E, Schauer F, Gesell Salazar M, Hessel S, Juelich WD, Lindequist U (2007) Novel cephalosporins synthesized by amination of 2,5-dihydroxybenzoic acid derivatives using fungal laccases II. Chem Pharm Bull 55(3):412–416. doi:10.1248/cpb.55.412
Mikolasch A, Hessel S, Gesell Salazar M, Neumann H, Manda K, Goerdes D, Schmidt E, Thurow K, Hammer E, Lindequist U, Beller M, Schauer F (2008a) Synthesis of new N-analogous corollosporine derivatives with antibacterial activity by laccase-catalyzed amination. Chem Pharm Bull 56(6):781–786. doi:10.1248/cpb.56.781
Mikolasch A, Matthies A, Lalk M, Schauer F (2008b) Laccase-induced C-N coupling of substituted p-hydroquinones with p-aminobenzoic acid in comparison with known chemical routes. Appl Microbiol Biotechnol 80(3):389–397. doi:10.1007/s00253-008-1595-y
Mikolasch A, Wurster M, Lalk M, Witt S, Seefeldt S, Hammer E, Schauer F, Juelich WD, Lindequist U (2008c) Novel β-lactam antibiotics synthesized by amination of catechols using fungal laccase. Chem Pharm Bull 56(7):902–907. doi:10.1248/cpb.56.902
Mikolasch A, Manda K, Schlueter R, Lalk M, Witt S, Seefeldt S, Hammer E, Schauer F, Juelich WD, Lindequist U (2012) Comparative analyses of laccase-catalyzed amination reactions for production of novel β-lactam antibiotics. Biotechnol Appl Biochem 59(4):295–306. doi:10.1002/bab.1026
Niedermeyer THJ, Mikolasch A, Lalk M (2005) Nuclear amination catalyzed by fungal laccases: reaction products of p-hydroquinones and primary aromatic amines. J Org Chem 70(6):2002–2008. doi:10.1021/jo048454s
Schultz A, Jonas U, Hammer E, Schauer F (2001) Dehalogenation of chlorinated hydroxybiphenyls by fungal laccase. Appl Environ Microbiol 67(9):4377–4381. doi:10.1128/aem.67.9.4377-4381.2001
Shimizu M, Shiota S, Mizushima T, Ito H, Hatano T, Yoshida T, Tsuchiya T (2001) Marked potentiation of activity of β-lactams against methicillin-resistant Staphylococcus aureus by corilagin. Antimicrob Agents Chemother 45(11):3198–3201. doi:10.1128/aac.45.11.3198-3201.2001
Tatsumi K, Freyer A, Minard RD, Bollag JM (1994) Enzyme-mediated coupling of 3,4-dichloroanilin and ferulic acid: a model for pollutant binding to humic materials. Environ Sci Technol 28(2):210–215. doi:10.1021/es00051a005
Thurston CF (1994) The structure and function of fungal laccases. Microbiol-Sgm 140:19–26
Waley SG (1992) ß-Lactamase: mechanism of action. In: Page MI (ed) The chemistry of ß-lactams. Blackie A. & P, London, pp 198–228
Yaropolov AI, Skorobogatko OV, Vartanov SS, Varfolomeyev SD (1994) Laccase—properties, catalytic mechanism, and applicability. Appl Biochem Biotechnol 49(3):257–280. doi:10.1007/bf02783061
Acknowledgments
We thank Robert Jack for the help in preparing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received.
Conflict of interest
All authors declare that they have no competing interests.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed.
This article does not contain any studies with human participants performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1165 kb)
Rights and permissions
About this article
Cite this article
Mikolasch, A., Hildebrandt, O., Schlüter, R. et al. Targeted synthesis of novel β-lactam antibiotics by laccase-catalyzed reaction of aromatic substrates selected by pre-testing for their antimicrobial and cytotoxic activity. Appl Microbiol Biotechnol 100, 4885–4899 (2016). https://doi.org/10.1007/s00253-016-7288-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7288-z