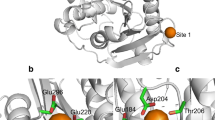
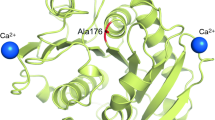
Abstract
Cutinases are esterases of industrial importance for applications in recycling and surface modification of polyesters. The cutinase from Thielavia terrestris (TtC) is distinct in terms of its ability to retain its stability and activity in acidic pH. Stability and activity in acidic pHs are desirable for esterases as the pH of the reaction tends to go down with the generation of acid. The pH stability and activity are governed by the charged state of the residues involved in catalysis or in substrate binding. In this study, we performed the detailed structural and biochemical characterization of TtC coupled with surface charge analysis to understand its acidic tolerance. The stability of TtC in acidic pH was rationalized by evaluating the contribution of charge interactions to the Gibbs free energy of unfolding at varying pHs. The activity of TtC was found to be limited by substrate binding affinity, which is a function of the surface charge. Additionally, the presence of glycosylation affects the biochemical characteristics of TtC owing to steric interactions with residues involved in substrate binding.










Similar content being viewed by others
References
Badenes SM, Lemos F, Cabral JMS (2010) Transesterification of oil mixtures catalyzed by microencapsulated cutinase in reversed micelles. Biotechnol Lett 32:399–403
Baker PJ, Poultney C, Liu Z, Gross R, Montclare JK (2012) Identification and comparison of cutinases for synthetic polyester degradation. Appl Microbiol Biotechnol 93:229–240
Banás P, Walter NG, Sponer J, Otyepka M (2010) Protonation states of the key active site residues and structural dynamics of the glmS riboswitch as revealed by molecular dynamics. J Phys Chem B 114:8701–8712
Beckham GT, Dai Z, Matthews JF, Momany M, Payne CM, Adney WS, Baker SE, Himmel ME (2012) Harnessing glycosylation to improve cellulase activity. Curr Opin Biotechnol 23:338–345
Bhardwaj H, Gupta R, Tiwari A (2012) Communities of microbial enzymes associated with biodegradation of plastics. J Polym Environ 21:575–579
Bommarius AS, Sohn M, Kang Y, Lee JH, Realff MJ (2014) Protein engineering of cellulases. Curr Opin Biotechnol 29:139–145
Bretthauer RK, Castellino FJ (1999) Glycosylation of Pichia pastoris-derived proteins. Biotechnol Appl Biochem 30(Pt 3):193–200
Chan C-H, Wilbanks CC, Makhatadze GI, Wong K-B (2012) Electrostatic contribution of surface charge residues to the stability of a thermophilic protein: benchmarking experimental and predicted pKa values. PLoS One 7:e30296
Chen L, Drake MR, Resch MG, Greene ER, Himmel ME, Chaffey PK, Beckham GT, Tan Z (2014) Specificity of O-glycosylation in enhancing the stability and cellulose binding affinity of Family 1 carbohydrate-binding modules. Proc Natl Acad Sci U S A 111:7612–7617
Chen S, Su L, Chen J, Wu J (2013) Cutinase: characteristics, preparation, and application. Biotechnol Adv 31:1754–1767
De Barros DPC, Azevedo AM, Cabral JMS, Fonseca LP (2012) Optimization of flavor esters synthesis by Fusarium solani pisi cutinase. J Food Biochem 36:275–284
Dutta K, Sen S, Veeranki VD (2009) Production, characterization and applications of microbial cutinases. Process Biochem 44:127–134
Fox JM, Levine SE, Clark DS, Blanch HW (2012) Initial- and processive-cut products reveal cellobiohydrolase rate limitations and the role of companion enzymes. Biochemistry 51:442–452
Gao D, Chundawat SPS, Sethi A, Balan V, Gnanakaran S, Dale BE (2013) Increased enzyme binding to substrate is not necessary for more efficient cellulose hydrolysis. Proc Natl Acad Sci U S A 110:10922–10927
García-Mayoral MF, del Pozo AM, Campos-Olivas R, Gavilanes JG, Santoro J, Rico M, Laurents DV, Bruix M (2006) pH-Dependent conformational stability of the ribotoxin alpha-sarcin and four active site charge substitution variants. Biochemistry 45:13705–13718
Gribenko AV, Patel MM, Liu J, SA MC, Wang C, Makhatadze GI (2009) Rational stabilization of enzymes by computational redesign of surface charge-charge interactions. Proc Natl Acad Sci U S A 106:2601–2606
Guebitz GM, Cavaco-Paulo A (2008) Enzymes go big: surface hydrolysis and functionalization of synthetic polymers. Trends Biotechnol 26:32–38
Guo M, Hang H, Zhu T, Zhuang Y, Chu J, Zhang S (2008) Effect of glycosylation on biochemical characterization of recombinant phytase expressed in Pichia pastoris. Enzym Microb Technol 42:340–345
Herrero Acero E, Ribitsch D, Dellacher A, Zitzenbacher S, Marold A, Steinkellner G, Gruber K, Schwab H, Guebitz GM (2013) Surface engineering of a cutinase from Thermobifida cellulosilytica for improved polyester hydrolysis. Biotechnol Bioeng 110:2581–2590
Hiraishi T, Komiya N, Maeda M (2010a) Y443F mutation in the substrate-binding domain of extracellular PHB depolymerase enhances its PHB adsorption and disruption abilities. Polym Degrad Stab 95:1370–1374
Hiraishi T, Komiya N, Matsumoto N, Abe H, Fujita M, Maeda M (2010b) Degradation and adsorption characteristics of PHB depolymerase as revealed by kinetics of mutant enzymes with amino acid substitution in substrate-binding domain. Biomacromolecules 11:113–119
Hlady V, Buijs J (1996) Protein adsorption on solid surfaces. Curr Opin Biotechnol 7:72–77
Horng J-C, Cho J-H, Raleigh DP (2005) Analysis of the pH-dependent folding and stability of histidine point mutants allows characterization of the denatured state and transition state for protein folding. J Mol Biol 345:163–173
Hu X, Thumarat U, Zhang X, Tang M, Kawai F (2010) Diversity of polyester-degrading bacteria in compost and molecular analysis of a thermoactive esterase from Thermobifida alba AHK119. Appl Microbiol Biotechnol 87:771–779
Hunsen M, Azim A, Mang H, Wallner SR, Ronkvist A, Xie W, Gross RA (2007) A cutinase with polyester synthesis activity. Macromolecules 40:148–150
Jehle S, Rajagopal P, Bardiaux B, Markovic S, Kühne R, Stout JR, Higman VA, Klevit RE, van Rossum B-J, Oschkinat H (2010) Solid-state NMR and SAXS studies provide a structural basis for the activation of alphaB-crystallin oligomers. Nat Struct Mol Biol 17:1037–1042
Kawai F, Oda M, Tamashiro T, Waku T, Tanaka N, Yamamoto M, Mizushima H, Miyakawa T, Tanokura M (2014) A novel Ca(2+)-activated, thermostabilized polyesterase capable of hydrolyzing polyethylene terephthalate from Saccharomonospora viridis AHK190. Appl Microbiol Biotechnol 98:10053–10064
Kelly SM, Jess TJ, Price NC (2005) How to study proteins by circular dichroism. Biochim Biophys Acta 1751:119–139
Khan MIM, Sajjad M, Sadaf S, Zafar R, Niazi UHK, Akhtar MW (2013) The nature of the carbohydrate binding module determines the catalytic efficiency of xylanase Z of Clostridium thermocellum. J Biotechnol 168:403–408
Kikkawa Y, Yamashita K, Hiraishi T, Kanesato M, Doi Y (2005) Dynamic adsoption behavior of poly(3-hydroxybutyrate) depolymerase onto polyester surface investigated by QCM and AFM. Biomacromolecules 6:2084–2090
Köller W, Kolattukudy PE (1982) Mechanism of action of cutinase: chemical modification of the catalytic triad characteristic for serine hydrolases. Biochemistry 21:3083–3090
Lin-Cereghino J, Wong WW, Xiong S, Giang W, Luong LT, Vu J, Johnson SD, Lin-Cereghino GP (2005) Condensed protocol for competent cell preparation and transformation of the methylotrophic yeast Pichia pastoris. Biotechniques 38:44, 46, 48
Liu Z, Gosser Y, Baker PJ, Ravee Y, Lu Z, Alemu G, Li H, Butterfoss GL, Kong X-P, Gross R, Montclare JK (2009) Structural and functional studies of Aspergillus oryzae cutinase: enhanced thermostability and hydrolytic activity of synthetic ester and polyester degradation. J Am Chem Soc 131:15711–15716
Martinez C, De Geus P, Lauwereys M, Matthyssens G, Cambillau C (1992) Fusarium solani cutinase is a lipolytic enzyme with a catalytic serine accessible to solvent. Nature 356:615–618
Martinez C, Nicolas A, van Tilbeurgh H, Egloff MP, Cudrey C, Verger R, Cambillau C (1994) Cutinase, a lipolytic enzyme with a preformed oxyanion hole. Biochemistry 33:83–89
Matamá T, Araújo R, Gübitz GM, Casal M, Cavaco-Paulo A (2009) Functionalization of cellulose acetate fibers with engineered cutinases. Biotechnol Prog 26:636–643
Mazzini A, Polverini E, Parisi M, Sorbi RT, Favilla R (2007) Dissociation and unfolding of bovine odorant binding protein at acidic pH. J Struct Biol 159:82–91. doi:10.1016/j.jsb.2007.02.007
Mueller R-J (2006) Biological degradation of synthetic polyesters—enzymes as potential catalysts for polyester recycling. Process Biochem 41:2124–2128
Neves-Petersen MT, Petersen EI, Fojan P, Noronha M, Madsen RG, Petersen SB (2001) Engineering the pH-optimum of a triglyceride lipase: from predictions based on electrostatic computations to experimental results. J Biotechnol 87:225–254
Nyyssölä A, Pihlajaniemi V, Häkkinen M, Kontkanen H, Saloheimo M, Nakari-Setälä T (2014) Cloning and characterization of a novel acidic cutinase from Sirococcus conigenus. Appl Microbiol Biotechnol 98:3639–3650
Nyyssölä A, Pihlajaniemi V, Järvinen R, Mikander S, Kontkanen H, Kruus K, Kallio H, Buchert J (2013) Screening of microbes for novel acidic cutinases and cloning and expression of an acidic cutinase from Aspergillus niger CBS 513.88. Enzyme and Microbial Technology 52:272–278
Payne CM, Resch MG, Chen L, Crowley MF, Himmel ME, Taylor LE, Sandgren M, Ståhlberg J, Stals I, Tan Z, Beckham GT (2013) Glycosylated linkers in multimodular lignocellulose-degrading enzymes dynamically bind to cellulose. Proc Natl Acad Sci U S A 110:14646–14651
Purdy RE, Kolattukudy PE (1975) Hydrolysis of plant cuticle by plant pathogens. Properties of cutinase I, cutinase II, and a nonspecific esterase isolated from Fusarium solani pisi. Biochemistry 14:2832–2840
Rhee JK, Ahn DG, Kim YG, Oh JW (2005) New thermophilic and thermostable esterase with sequence similarity to the hormone-sensitive lipase family, cloned from a metagenomic library. Appl Environ Microbiol 71:817–825
Ronkvist ÅM, Lu W, Feder D, Gross RA (2009a) Cutinase-catalyzed deacetylation of poly(vinyl acetate). Macromolecules 42:6086–6097
Ronkvist ÅM, Xie W, Lu W, Gross RA (2009b) Cutinase-catalyzed hydrolysis of poly(ethylene terephthalate). Macromolecules 42:5128–5138
Roth C, Wei R, Oeser T, Then J, Föllner C, Zimmermann W, Sträter N (2014) Structural and functional studies on a thermostable polyethylene terephthalate degrading hydrolase from Thermobifida fusca. Appl Microbiol Biotechnol 98:7815–7823
Sampogna RV, Honig B (1994) Environmental effects on the protonation states of active site residues in bacteriorhodopsin. Biophys J 66:1341–1352
Scandola M, Focarete ML, Frisoni G (1998) Simple kinetic model for the heterogeneous enzymatic hydrolysis of natural poly (3-hydroxybutyrate). Macromolecules 31:3846–3851
Skropeta D (2009) The effect of individual N-glycans on enzyme activity. Bioorganic Med Chem 17:2645–2653
Strickler SS, Gribenko AV, Gribenko AV, Keiffer TR, Tomlinson J, Reihle T, Loladze VV, Makhatadze GI, Pennsyl V (2006) Accelerated publications protein stability and surface electrostatics : a charged relationship. Biochemistry 45(9):2761–1766
Stryer L (1968) Fluorescence spectroscopy of proteins. Science 162:526–533
Sulaiman S, Yamato S, Kanaya E, Kim J-J, Koga Y, Takano K, Kanaya S (2012) Isolation of a novel cutinase homolog with polyethylene terephthalate-degrading activity from leaf-branch compost by using a metagenomic approach. Appl Environ Microbiol 78:1556–1562
Sulaiman S, You D-J, Kanaya E, Koga Y, Kanaya S (2014) Crystal structure and thermodynamic and kinetic stability of metagenome-derived LC-cutinase. Biochemistry 53:1858–1869
Sussman F, Villaverde CM, Dominguez JL, Danielson UH (2013) On the active site protonation state in aspartic proteases: implications for drug design. Curr Pharm Des 19(23):4257–4275
Talley K, Alexov E (2010) On the pH-optimum of activity and stability of proteins. Proteins 78:2699–2706
Wang C, Eufemi M, Turano C, Giartosio A (1996) Influence of the carbohydrate moiety on the stability of glycoproteins. Biochemistry 35:7299–7307
Yamato I, Rosenbusch JP (1983) Dependence on pH of substrate binding to lactose carrier in Escherichia coli cytooplasmic membranes. FEBS Lett 151:102–104
Yang S, Xu H, Yan Q, Liu Y, Zhou P, Jiang Z (2013) A low molecular mass cutinase of Thielavia terrestris efficiently hydrolyzes poly(esters). J Ind Microbiol Biotechnol 40:217–226
Zacharius RM, Zell TE, Morrison JH, Woodlock JJ (1969) Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem 30:148–152
Zhu J, Liu H, Zhang J, Wang P, Liu S, Liu G, Wu L (2014) Effects of Asn-33 glycosylation on the thermostability of Thermomyces lanuginosus lipase. J Appl Microbiol 117:151–159
Acknowledgments
This work was supported by the National Science Foundation’s Chemical, Bioengineering, Environmental and Transport Systems (CBET) Division (Award # 1067415) to R.A.G. and NIH grant R01 GM099827 to C.B. We thank DNA2.0 for gene synthesis and helpful suggestions during the course of this work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 412 kb)
Rights and permissions
About this article
Cite this article
Shirke, A.N., Basore, D., Holton, S. et al. Influence of surface charge, binding site residues and glycosylation on Thielavia terrestris cutinase biochemical characteristics. Appl Microbiol Biotechnol 100, 4435–4446 (2016). https://doi.org/10.1007/s00253-015-7254-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-7254-1