Abstract
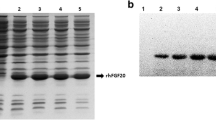
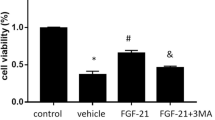
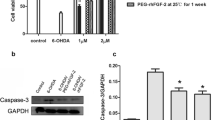
Human fibroblast growth factor 8b (FGF8b) was expressed based on a baculovirus expression vector system (BEVS) and identified as having a protective effect on Parkinson’s disease. Immunoblotting demonstrated that rhFGF8b proteins were recognized by a human anti-FGF8b antibody. The multiplicity of infection and timing of harvest had a significant effect on protein yield and protein quality. Our results indicated that the rhFGF8b was first detectable at 36 h postinfection and reached a maximum at 60 h. A multiplicity of infection (MOI) of 8 pfu/mL was suitable for harvest. The target protein was purified by heparin-affinity chromatography. In vitro methylthiazol tetrazolium (MTT) assays demonstrated that the purified rhFGF8b could significantly stimulate proliferation of NIH3T3 cells. Furthermore, to elucidate the effect of rhFGF8b on Parkinson’s disease, we used FGF8b pretreatment on a cell model of Parkinson’s disease. The results indicated that rhFGF8b prevented necrosis and apoptosis of 1-METHYL-4-phenyl pyridine (MPP+) treated PC12 cells. Moreover, the effect of FGF8b on messenger RNA (mRNA) levels of apoptosis and ERS genes was investigated to clarify the molecular mechanisms of FGF8b. The results suggest that FGF8b exerts neuroprotective effects by alleviating endoplasmic reticulum (ER) stress during PD. These results suggest that FGF8b may be a promising candidate therapeutic drug for neurodegenerative diseases related to ER stress.






Similar content being viewed by others
References
Aarsland D, Pahlhagen S, Ballard CG, Ehrt U, Svenningsson P (2012) Depression in Parkinson disease—epidemiology, mechanisms and management. Nat Rev Neurol 8(1):35–47. doi:10.1038/nrneurol.2011.189
Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM, Trojanowski JQ, Iwatsubo T (1998) Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am J Pathol 152(4):879–884
Berman B, Ostrovsky O, Shlissel M, Lang T, Regan D, Vlodavsky I, Ishai-Michaeli R, Ron D (1999) Similarities and differences between the effects of heparin and glypican-1 on the bioactivity of acidic fibroblast growth factor and the keratinocyte growth factor. J Biol Chem 274(51):36132–36138
Carinhas N, Bernal V, Yokomizo AY, Carrondo MJ, Oliveira R, Alves PM (2009) Baculovirus production for gene therapy: the role of cell density, multiplicity of infection and medium exchange. Appl Microbiol Biotechnol 81(6):1041–1049. doi:10.1007/s00253-008-1727-4
Crossley PH, Martin GR (1995) The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development 121(2):439–451
de Oliveira GP, Duobles T, Castelucci P, Chadi G (2010) Differential regulation of FGF-2 in neurons and reactive astrocytes of axotomized rat hypoglossal nucleus. A possible therapeutic target for neuroprotection in peripheral nerve pathology. Acta Histochem 112(6):604–617. doi:10.1016/j.acthis.2009.06.008
Dong X, Tang B, Li J, Xu Q, Fang S, Hua Z (2008) Expression and purification of intact and functional soybean (Glycine max) seed ferritin complex in Escherichia coli. J Microbiol Biotechnol 18(2):299–307
Drugmand JC, Schneider YJ, Agathos SN (2012) Insect cells as factories for biomanufacturing. Biotechnol Adv 30(5):1140–1157. doi:10.1016/j.biotechadv.2011.09.014
Gemel J, Gorry M, Ehrlich GD, MacArthur CA (1996) Structure and sequence of human FGF8. Genomics 35(1):253–257. doi:10.1006/geno.1996.0349
Ghosh AK, Shankar DB, Shackleford GM, Wu K, T’Ang A, Miller GJ, Zheng J, Roy-Burman P (1996) Molecular cloning and characterization of human FGF8 alternative messenger RNA forms. Cell Growth Differ 7(10):1425–1434
Gnanapragasam VJ, Robinson MC, Marsh C, Robson CN, Hamdy FC, Leung HY (2003) FGF8 isoform b expression in human prostate cancer. Br J Cancer 88(9):1432–1438. doi:10.1038/sj.bjc.6600875
Heikinheimo M, Lawshe A, Shackleford GM, Wilson DB, MacArthur CA (1994) Fgf-8 expression in the post-gastrulation mouse suggests roles in the development of the face, limbs and central nervous system. Mech Dev 48(2):129–138
Hetz C, Chevet E, Harding HP (2013) Targeting the unfolded protein response in disease. Nat Rev Drug Discov 12(9):703–719. doi:10.1038/nrd3976
Huang Z, Ye C, Liu Z, Wang X, Chen H, Liu Y, Tang L, Zhao H, Wang J, Feng W, Li X (2012) Solid-phase N-terminus PEGylation of recombinant human fibroblast growth factor 2 on Heparin-Sepharose column. Bioconjug Chem 23(4):740–750. doi:10.1021/bc200550f
Huang P, Wang Z, Tian H, Zhao H, Li H, X L (2013) The constructing and purification of recombinant human fibroblast growth factor 8b expressed vector.pdf. China Biotechnology 33(1):14-19
Kenig M, Gaberc-Porekar V, Fonda I, Menart V (2008) Identification of the heparin-binding domain of TNF-alpha and its use for efficient TNF-alpha purification by Heparin-Sepharose affinity chromatography. J Chromatogr B Analyt Technol Biomed Life Sci 867(1):119–125. doi:10.1016/j.jchromb.2008.03.023
Kwabi-Addo B, Ozen M, Ittmann M (2004) The role of fibroblast growth factors and their receptors in prostate cancer. Endocr Relat Cancer 11(4):709–724. doi:10.1677/erc.1.00535
Lindholm D, Wootz H, Korhonen L (2006) ER stress and neurodegenerative diseases. Cell Death Differ 13(3):385–392. doi:10.1038/sj.cdd.4401778
Litteljohn D, Hayley S (2012) Cytokines as potential biomarkers for Parkinson’s disease: a multiplex approach. Methods Mol Biol 934:121–144. doi:10.1007/978-1-62703-071-7_7
Liu H, Bowes RC 3rd, van de Water B, Sillence C, Nagelkerke JF, Stevens JL (1997) Endoplasmic reticulum chaperones GRP78 and calreticulin prevent oxidative stress, Ca2+ disturbances, and cell death in renal epithelial cells. J Biol Chem 272(35):21751–21759
Lorenzi MV, Long JE, Miki T, Aaronson SA (1995) Expression cloning, developmental expression and chromosomal localization of fibroblast growth factor-8. Oncogene 10(10):2051–2055
Mahmood R, Bresnick J, Hornbruch A, Mahony C, Morton N, Colquhoun K, Martin P, Lumsden A, Dickson C, Mason I (1995) A role for FGF-8 in the initiation and maintenance of vertebrate limb bud outgrowth. Curr Biol 5(7):797–806
Mattila MM, Ruohola JK, Valve EM, Tasanen MJ, Seppanen JA, Harkonen PL (2001) FGF-8b increases angiogenic capacity and tumor growth of androgen-regulated S115 breast cancer cells. Oncogene 20(22):2791–2804. doi:10.1038/sj.onc.1204430
Ohuchi H, Yoshioka H, Tanaka A, Kawakami Y, Nohno T, Noji S (1994) Involvement of androgen-induced growth factor (FGF-8) gene in mouse embryogenesis and morphogenesis. Biochem Biophys Res Commun 204(2):882–888. doi:10.1006/bbrc.1994.2542
Omura T, Asari M, Yamamoto J, Kamiyama N, Oka K, Hoshina C, Maseda C, Awaya T, Tasaki Y, Shiono H, Shimizu K, Matsubara K (2012) HRD1 levels increased by zonisamide prevented cell death and caspase-3 activation caused by endoplasmic reticulum stress in SH-SY5Y cells. J Mol Neurosci 46(3):527–535. doi:10.1007/s12031-011-9638-8
Payson RA, Wu J, Liu Y, Chiu IM (1996) The human FGF-8 gene localizes on chromosome 10q24 and is subjected to induction by androgen in breast cancer cells. Oncogene 13(1):47–53
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):e45
Pluquet O, Pourtier A, Abbadie C (2015) The unfolded protein response and cellular senescence. A review in the theme: cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. Am J Physiol Cell Physiol 308(6):C415–C425. doi:10.1152/ajpcell.00334.2014
Potula HH, Kathuria SR, Ghosh AK, Maiti TK, Dey S (2008) Transient expression, purification and characterization of bioactive human fibroblast growth factor 8b in tobacco plants. Transgenic Res 17(1):19–32. doi:10.1007/s11248-007-9072-4
Rodriguez-Oroz MC, Jahanshahi M, Krack P, Litvan I, Macias R, Bezard E, Obeso JA (2009) Initial clinical manifestations of Parkinson’s disease: features and pathophysiological mechanisms. Lancet Neurol 8(12):1128–1139. doi:10.1016/s1474-4422(09)70293-5
Roussa E, Krieglstein K (2004) Induction and specification of midbrain dopaminergic cells: focus on SHH, FGF8, and TGF-beta. Cell Tissue Res 318(1):23–33. doi:10.1007/s00441-004-0916-4
Schapira AH, Olanow CW (2004) Neuroprotection in Parkinson disease: mysteries, myths, and misconceptions. JAMA 291(3):358–364. doi:10.1001/jama.291.3.358
Shrivastava P, Vaibhav K, Tabassum R, Khan A, Ishrat T, Khan MM, Ahmad A, Islam F, Safhi MM, Islam F (2013) Anti-apoptotic and anti-inflammatory effect of Piperine on 6-OHDA induced Parkinson’s rat model. J Nutr Biochem 24(4):680–687. doi:10.1016/j.jnutbio.2012.03.018
Sun X, Meyers EN, Lewandoski M, Martin GR (1999) Targeted disruption of Fgf8 causes failure of cell migration in the gastrulating mouse embryo. Genes Dev 13(14):1834–1846
Sun C, Li Y, Taylor SE, Mao X, Wilkinson MC, Fernig DG (2015) HaloTag is an effective expression and solubilisation fusion partner for a range of fibroblast growth factors. Peer J 3 doi:10.7717/peerj.1060
Tanaka A, Miyamoto K, Minamino N, Takeda M, Sato B, Matsuo H, Matsumoto K (1992) Cloning and characterization of an androgen-induced growth factor essential for the androgen-dependent growth of mouse mammary carcinoma cells. Proc Natl Acad Sci U S A 89(19):8928–8932
Tanaka A, Furuya A, Yamasaki M, Hanai N, Kuriki K, Kamiakito T, Kobayashi Y, Yoshida H, Koike M, Fukayama M (1998) High frequency of fibroblast growth factor (FGF) 8 expression in clinical prostate cancers and breast tissues, immunohistochemically demonstrated by a newly established neutralizing monoclonal antibody against FGF 8. Cancer Res 58(10):2053–2056
Tanaka A, Kamiakito T, Hakamata Y, Fujii A, Kuriki K, Fukayama M (2001) Extensive neuronal localization and neurotrophic function of fibroblast growth factor 8 in the nervous system. Brain Res 912(2):105–115
Trowitzsch S, Bieniossek C, Nie Y, Garzoni F, Berger I (2010) New baculovirus expression tools for recombinant protein complex production. J Struct Biol 172(1):45–54. doi:10.1016/j.jsb.2010.02.010
Uchii M, Tamura T, Suda T, Kakuni M, Tanaka A, Miki I (2008) Role of fibroblast growth factor 8 (FGF8) in animal models of osteoarthritis. Arthritis Res Ther 10(4):R90. doi:10.1186/ar2474
Volmer R, van der Ploeg K, Ron D (2013) Membrane lipid saturation activates endoplasmic reticulum unfolded protein response transducers through their transmembrane domains. Proc Natl Acad Sci U S A 110(12):4628–4633. doi:10.1073/pnas.1217611110
Ye W, Shimamura K, Rubenstein JL, Hynes MA, Rosenthal A (1998) FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell 93(5):755–766
Yoshida H (2007) ER stress and diseases. FEBS J 274(3):630–658. doi:10.1111/j.1742-4658.2007.05639.x
Zhong C, Saribekyan G, Liao CP, Cohen MB, Roy-Burman P (2006) Cooperation between FGF8b overexpression and PTEN deficiency in prostate tumorigenesis. Cancer Res 66(4):2188–2194. doi:10.1158/0008-5472.can-05-3440
Acknowledgments
The project was supported by Zhejiang Extremely Key Subject of Pharmacology and Biochemical Pharmaceutics and the National “863” High Technology Research and Development Program (2011AA02A113).
Compliance with Ethical Standards
ᅟ
Conflict of Interest
The authors declare that they have no competing interests.
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chen, N., Ma, J., Zhao, Y. et al. Expression of functional recombinant human fibroblast growth factor 8b and its protective effects on MPP+-lesioned PC12 cells. Appl Microbiol Biotechnol 100, 625–635 (2016). https://doi.org/10.1007/s00253-015-7004-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-7004-4