Abstract
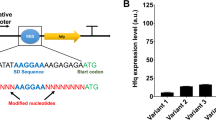
For making a complex synthetic gene network function as designed, the parameters in the network have to be extensively tuned. In this study, a simple and general approach to rapidly tune gene networks in Escherichia coli was developed, which uses the hypermutable simple sequence repeats embedded in the spacer region between the ribosome binding site and the initiation codon. It was found that the change of sequence length and compositions of the repeated base pairs in 5′UTR contributes together to the changeable expression levels of the target gene. The mechanism of this phenomenon is that the transcriptional process makes greater impact on the expression level when compared to the translational process, which is utilized to successfully predict sample gene expression levels over a 50-fold range. The utility of the approach to regulate heterologous ecaA and pepc gene expression in the engineered E. coli for improving succinic acid yield and production has also been demonstrated. When the expression level of ecaA gene was 3.53-fold of the control and the expression level of pepc gene was 1.06-fold of the control, the highest yield of succinic acid and productivity were achieved, which was 0.87 g g−1 and 2.01 g L−1 h−1, respectively.



Similar content being viewed by others
References
Alper H, Fischer C, Nevoigt E, Setphanopoulos G (2005) Tuning genetic control through promoter engineering. Proc Natl Acad Sci U S A 102:12678–12683
Badger MR, Price GD (2003) CO2 concentrating mechanism in cyanobacteria: molecular components, their diversity and evolution. J Exp Bot 54:609–622
Bailey JE (1991) Toward a science of metabolic engineering. Science 252:1668–1675
Balzer GJ, Thakker C, Bennett GN, San KY (2013) Metabolic engineering of Escherichia coli to minimize byproduct formate and improving succinate productivity through increasing NADH availability by heterologous expression of NAD(+)-dependent formate dehydrogenase. Metab Eng 20:1–8
Barrick D, Villanueba K, Childs J, Kalil R, Schneider TD, Lawrence CE, Gold L, Stormo GD (1994) Quantitative-analysis of ribosome binding-sites in Escherichia coli. Nucleic Acids Res 22:1287–1295
Beato M (1989) Gene-regulation by steroid-hormones. Cell 56:335–344
Benner SA, Sismour AM (2005) Synthetic biology. Nat Rev Genet 6:533–543
Bi CH, Su P, Muller J, Yeh YC, Chhabra SR, Beller HR, Singer SW, Hillson NJ (2013) Development of a broad-host synthetic biology toolbox for Ralstonia eutropha and its application to engineering hydrocarbon biofuel production. Microb Cell Factories 12:107
Callura JM, Dwyer DJ, Isaacs FJ, Cantor CR, Collins JJ (2010) Tracking, tuning, and terminating microbial physiology using synthetic riboregulators. Proc Natl Acad Sci U S A 107:15898–15503
Chen H, Bijerknes M, Kumar R, Jay E (1994) Determination of the optimal aligned spacing between the Shine-Dalgarno sequence and the translation initiation codon of Escherichia coli mRNAs. Nucleic Acids Res 22:4953–4957
Clark DP (1989) The fermentation pathways of Escherichia coli. FEMS Microbiol Rev 63:223–234
Cox RS, Surette MG, Elowitz MB (2007) Programming gene expression with combinatorial promoters. Mol Syst Biol 3:145
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad U S A 97(12):6640–6645
Dong HJ, Tao WW, Zhang YP, Li Y (2012) Development of an anhydrotetracycline-inducible gene expression system for solvent-producing Clostridium acetobutylicum: a useful tool for strain engineering. Metab Eng 14:59–67
Egbert RG, Klavins E (2012) Fine-tuning gene networks using simple sequence repeats. Proc Natl Acad Sci USA 109:16817–16822
Eiteman MA, Miller JH (1995) Effect of succinic acid on 2,3-butanediol production by Klebsiella oxytoca. Biotechnol Lett 17:1057–1062
Feist AM, Palsson BO (2008) The growing scope of applications of genome-scale metabolic reconstructions using Escherichia coli. Nat Biotechnol 26:659–667
Fischbach M, Voigt CA (2010) Prokaryotic gene clusters: a rich toolbox for synthetic biology. Biotechnol J 5:1277–1296
Gibson DG, Glass JI, Lartigue C, Noskov VN, Chuang RY, Algire MA, Benders GA, Montague MG, Ma L, Moodie MM, Merryman C, Vashee S, Krishnakumar R, Assas-Garcia N, Andrews-Pfannkoch C, Denisova EA, Young L, Qi ZQ, Segall-Shapiro TH, Calvey CH, Parmar PP, Hutchison CA, Smith HO, Venter JC (2010) Creation of a bacterial cell controlled by a chemically synthesized genome. Science 329:52–56
Hawkins AS, McTernan PM, Lian H, Kelly RM, Adams MWW (2013) Biological conversion of carbon dioxide and hydrogen into liquid fuels and industrial chemicals. Curr Opin Biotechnol 24:376–384
Kai Y, Matsumura H, Lzui K (2003) Phosphoenolpyruvate carboxylase: three-dimensional structure and molecular mechanisms. Arch Biochem Biophys 414:170–179
Keasling JD (2010) Manufacturing molecules through metabolic engineering. Science 330:1355–1358
Kodaki T, Katagiri F, Asano M, Izui K, Katsuki H (1985) Cloning of phosphoenolpyruvate carboxylase gene from a cyanobacterium, Anacystis nidulans, in Escherichia coli. J Biochem 97:533–539
Kozliak EI, Fuchs JA, Guilloton MB, Anderson PM (1995) Role of bicarbonate/CO2 in the inhibition of Escherichia coli growth by cyanate. J Bacteriol 177:3213–3219
Laetitia M, Martin F (2006) Impact of RNA interference on gene networks. Metab Eng 8(6):672–683
Langer R, Tirrell DA (2004) Designing materials for biology and medicine. Nature 428:487–492
Lee JW, Na D, Park JM, Lee J, Choi S, Lee SY (2012) Systems metabolic engineering of microorganisms for natural and non-natural chemicals. Nat Chem Biol 8:536–546
Leitao AL, Engutia FJ (2014) Fungal extrolites as a new source for therapeutic compounds and as building blocks for applications in synthetic biology. Microbiol Res 169:652–665
Lescot M, Dehais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouze P, Rombauts S (2002) PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res 30:325–327
Levin-Karp A, Barenholz U, Bareia T, Dayagi M, Zelcbuch L, Antonovsky N, Noor E, Milo R (2013) Quantifying translational coupling in E. coli synthetic operons using RBS modulation and fluorescent reporters. ACS Synth Biol 2:327–336
Liang LY, Liu RM, Ma JF, Chen KQ, Jiang M, Wei P (2011) Increased production of succinic acid in Escherichia coli by overexpression of malate dehydrogenase. Biotechnol Lett 33:2439–2444
Liu YP, Zheng P, Sun ZH, Ni Y, Dong JJ, Zhu LL (2008) Economical succinic acid production from cane molasses by Actinobacillus succinogenes. Bioresour Technol 99:1736–1742
Liu RM, Liang LY, Wu MK, Chen KQ, Jiang M, Ma JF, Wei P, Ouyang PK (2013) CO2 fixation for succinic acid production by engineered Escherichia coli co-expressing pyruvate carboxylase and nicotinic acid phosphoribosyltransferase. Biochem Eng J 79:77–83
Lu TK, Khalil AS, Collins JJ (2009) Next-generation synthetic gene networks. Nat Biotechnol 27:1139–1150
Mayfield S (2013) The green revolution 20: the potential of algae for the production of biofuels and bioproducts. Genome 56:551–555
McKinlay JB, Vieille C, Zeikus JG (2007) Prospects for a bio-based succinate industry. Appl Microbiol Biotechnol 76:727–740
Millard CS, Chao Y-P, Liao JC, Donnelly MI (1996) Enhanced production of succinic acid by overexpression of phosphoenolpyruvate carboxylase in Escherichia coli. Appl Environ Microbiol 62:1808–1810
Mossman BT, Borm PJ, Castranova V, Costa DL, Donaldson K, Kleeberger SR (2007) Mechanisms of action of inhaled fibers, particles and nanoparticles in lung and cardiovascular diseases. Part Fibre Toxicol 4:4
Mutalik VK, Qi L, Guimaraes JC, Lucks JB, Arkin AP (2012) Rationally designed families of orthogonal RNA regulators of translation. Nat Chem Biol 8:447–454
Oliver JWK, Machado IMP, Yoneda H, Atsumi S (2014) Combinatorial optimization of cyanobacterial 2,3-butanediol production. Metab Eng 22:76–82
Orom UA, Nielsen FC, Lund AH (2008) MicroRNA-10a binds the 5′UTR of ribosomal protein mRNAs and enhances their translation. Mol Cell 30:460–471
Paddon CJ, Keasling JD (2014) Semi-synthetic artemisinin: a model for the use of synthetic biology in pharmaceutical development. Nat Rev Microbiol 12:355–367
Pribnow D (1975) Nucleotide-sequence of an RNA-polymerase binding-site at an early T7 promoter. Proc Natl Acad U S A 72:784–788
Price GD, Badger MR (1989) Expression of human carbonic anhydrase in the cyanobacterium Synechococcus PCC7942 creates a high CO2-requiring phenotype. Evidence for a central role for carboxysomes in the CO2 concentrating mechanism. Plant Physiol 91:505–513
Rabinovitch-Deere CA, Oliver JWK, Rodriguez GM, Atsumi S (2013) Synthetic biology and metabolic engineering approaches to produce biofuels. Chem Rev 113:4611–4632
Ro DK, Paradise EM, Ouellet M, Fisher KJ, Newman KL, Ndungu JM, Ho KA, Eachus RA, Ham TS, Kridy J (2006) Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 440:940–943
Sakai Y, Abe K, Nakashima S, Yoshida W, Ferri S, Sode K, Ikebukuro K (2014) Improving the gene-regulation ability of small RNAs by scaffold engineering in Escherichia coli. ACS Synth Biol 3:152–162
Salis HM, Mirsky EA, Voigt CA (2009) Automated design of synthetic ribosome binding sites to control protein expression. Nat Biotechnol 27:946–950
Sueel GM, Kulkarni RP, Dworkin J, Garcia-Ojalvo J, Elowtiz MB (2007) Tunability and noise dependence in differentiation dynamics. Science 315:1716–1719
Thakker C, Zhu JF, San KY, Bennet G (2011) Heterologous pyc gene expression under various natural and engineered promoters in Escherichia coli for improved succinate production. J Biotechnol 155:236–243
Tong AHY, Evangelista M, Parsons AB, Xu H, Bader GD, Page N, Robinson M, Raghibizadeh S, Hogue CWV, Bussey H, Andrews B, Tyers M, Boone C (2001) Systematic genetic analysis with ordered arrays of yeast deletion mutants. Science 294:2364–2368
Vellanoweth RL, Rabinowitz JC (1992) The influence of ribosome-binding-site elements on translational efficiency in Bacillus subtilis and Escherichia coli in vivo. Mol Microbiol 6:1105–1114
Wang D, Li Q, Li WL, Xing JM, Su ZG (2009) Improvement of succinic acid production by overexpression of a cyanobacterial carbonic anhydrase in Escherichia coli. Enzyme Microbial Technol 45:491–497
Wang D, Li Q, Mao Y, Xing J, Su Z (2010) High-level succinic acid production and yield by lactose-induced expression of phosphoenolpyruvate carboxylase in ptsG mutant Escherichia coli. Appl Microbiol Biotechnol 87:2025–2035
Wang C, Zhang HL, Cai H, Zhou ZH, Chen YL, Ouyang PK (2014a) Succinic acid production from corn cob hydrolysates by genetically engineering Corynebacterium glutamicum. Appl Biochem Biotechnol 172:340–350
Wang J, Zhang BY, Zhang J, Wang HH, Zhao MH, Wang N, Dong LC, Zhou XH, Wang D (2014b) Enhanced succinic acid production and magnesium utilization by overexpression of magnesium transporter mgtA in Escherichia coli mutant. Bioresour Technol 170:125–131
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16s ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
Werpy T, Petersen G (2004) Top value added chemicals from biomass. Department of energy. Washington, DC, pp 31–33
Wibur KM, Anderson NG (1948) Electrometric and colorimetric determination of carbonic anhydrase. J Biol Chem 176:147–154
Yang DD, Francois JM, de Billerbeck GM (2012) Cloning, expression and characterization of an aryl-alcohol dehydrogenase from the white-rot fungus Phanerochaete chrysosporium strain BKM-F-1767. BMC Microbiol 12:126
Ye VM, Bhatia SK (2012) Metabolic engineering for the production of clinically important molecules: omega-3 fatty acids, artemisinin, and taxol. Biotechnol J 7:20–33
Yeh PJ, Hegreness MJ, Aiden AP, Kishony R (2009) Systems Microbiology. Opinion: drug interactions and the evolution of antibiotic resistance. Nat Rev Microbiol 7:460–466
Zelcbuch L, Antonovsky N, Bar-Even A, Levin-Karp A, Barenholz U, Dayagi M, Liebermeister W, Flamholz A, Noor E, Amram S, Brandis A, Bareia T, Yofe I, Jubran H, Milo R (2013) Spanning high-dimensional expression space using ribosome-binding site combinatorics. Nucleic Acids Res 41:e98
Zhang XL, Jantama K, Moore JC, Jarboe LR, Shanmugam KT, Ingram LO (2009) Metabolic evolution of energy-conserving pathways for succinate production in Escherichia coli. Proc Natl Acad U S A 106:20180–20185
Zhang F, Carothers JM, Keasling JD (2012) Design of a dynamic sensor-regulator system for production of chemicals and fuels derived from fatty acids. Nat Biotechnol 30:354–359
Zhu LW, Li XH, Zhang L, Li HM, Liu JH, Yuan ZP, Chen T, Tang YJ (2013) Activation of glyoxylate pathway without the activation of its related gene in succinate-producing engineered Escherichia coli. Metab Eng 20:9–19
Acknowledgments
This research was supported by the National Natural Science Foundation of China (Grant 21106191, 21206175), the State Key Laboratory of Materials-Oriented Chemical Engineering (Grant KL14-11), and Fundamental Research Funds for the Central Universities (project no.CQDXWL-2013-019). The authors thank Professor Clark (Southern Illinois University) for strain DC1515.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
(DOC 58.5 kb)
Rights and permissions
About this article
Cite this article
Wang, J., Qin, D., Zhang, B. et al. Fine-tuning of ecaA and pepc gene expression increases succinic acid production in Escherichia coli . Appl Microbiol Biotechnol 99, 8575–8586 (2015). https://doi.org/10.1007/s00253-015-6734-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6734-7