Abstract
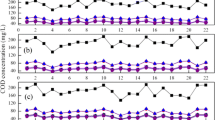
On the basis of achieving shortcut nitrification in a lab-scale SBR, the effects of constant pH and unsteady pH at different free ammonia concentrations on shortcut nitrification for landfill leachate treatment was investigated. The results indicate that under the condition of DO of 0.5 ± 0.2 mg/L and temperature of 30 ± 2 °C, the absolute value of nitrite accumulation increased significantly with the increase in free ammonia (FA) concentration from 5.30 to 48.67 mg/L; however, the nitrite accumulation rate remained almost constant at a constant pH of 8.0 ± 0.1. Ammonia oxidation and the nitrite accumulation become slow with the pH decreased from 8.0 ± 0.1 to 7.5 ± 0.2, and the activities of ammonia-oxidizing bacteria (AOB) and nitrite-oxidizing bacteria (NOB) were severely inhibited when the pH further decreased to 6.5. More importantly, this study confirmed that the pH decrease from 8.0 to 6.5 within a short time exhibited significant negative effect on the ammonia oxidation rather than the FA concentration.







Similar content being viewed by others
Reference
Anthonisen AC, Loehr RC, Prakasam TBS, Srinath EG (1976) Inhibition of nitrification by ammonia and nitrous acid. Water Pollut Control Fed 48:835–852
Bae W, Baek S, Chung J, Lee Y (2001) Optimal operational factors for nitrite accumulation in batch reactors. Biodegradation 12(5):359–366
Blackburne R, Yuan Z, Keller J (2008) Partial nitrification to nitrite using low dissolved oxygen concentration as the main selection factor. Biodegradation 19(2):303–312
Chinese N. E. P. A. (2002) Water and wastewater monitoring methods. Chinese Environmental Science Publishing House, Beijing, 354. (in Chinese)
Groeneweg J, Sellner B, Tappe W (1994) Ammonia oxidation in Nitrosomonas at NH3 concentrations near K m: effects of pH and temperature. Water Res 28(12):2561–2566
Guo J, Peng Y, Wang S, Zheng Y, Huang H, Wang Z (2009) Long-term effect of dissolved oxygen on partial nitrification performance and microbial community structure. Bioresour Technol 100(11):2796–2802
Hellinga C, Schellen AAJC, Mulder JW, Van Loosdrecht MCM, Heijnen JJ (1998) The SHARON process: an innovative method for nitrogen removal from ammonium-rich waste water. Water Sci Technol 37(9):135–142
Hellinga C, Van Loosdrecht MCM, Heijnen JJ (1999) Model based design of a novel process for nitrogen removal from concentrated flows. Math Comput Model Dyn 5(4):351–371
Hunik JH, Tramper J, Wijffels RH (1994) A strategy to scale up nitrification processes with immobilized cells of Nitrosomonas europaea and Nitrobacter agilis. Bioprocess Eng 11(2):73–82
Khin T, Annachhatre AP (2004) Novel microbial nitrogen removal processes. Biotechnol Adv 22(7):519–532
Kim JH, Guo X, Park HS (2008) Comparison study of the effects of temperature and free ammonia concentration on nitrification and nitrite accumulation. Process Biochem 43(2):154–160
Laanbroek HJ, Bodelier PL, Gerards S (1994) Oxygen consumption kinetics of Nitrosomonas europaea and Nitrobacter hamburgensis grown in mixed continuous cultures at different oxygen concentrations. Arch Microbiol 161(2):156–162
Li HS, Zhou SQ, Sun YB, Feng P, Li JD (2009) Advanced treatment of landfill leachate by a new combination process in a full-scale plant. J Hazard Mater 172(1):408–415
Morgenroth E, Obermayer A, Arnold E, Brl A, Wagner M, Wilderer P (2000) Effect of long-term idle periods on the performance of sequencing batch reactors. Water Sci Technol 41(1):105–113
Park S, Bae W, Rittmann BE (2009) Operational boundaries for nitrite accumulation in nitrification based on minimum/maximum substrate concentrations that include effects of oxygen limitation, pH, and free ammonia and free nitrous acid inhibition. Environ Sci Technol 44(1):335–342
Peng Y, Zhang S, Zheng S, Wang S (2006) Heavy metal ions in the biological treatment of municipal landfill leachate. Chin J Env Eng 7(1):1–5 (in Chinese)
Renou S, Givaudan JG, Poulain S, Dirassouyan F, Moulin P (2008) Landfill leachate treatment: review and opportunity. J Hazard Mater 150(3):468–493
Sri Shalini S, Joseph K (2012) Nitrogen management in landfill leachate: application of SHARON, ANAMMOX and combined SHARON–ANAMMOX Process. Waste Manag 32(12):2385–2400
Suzuki I, Dular U, Kwok SC (1974) Ammonia or ammonium ion as substrate for oxidation by Nitrosomonas europaea cells and extracts. J Bacteriol 120(1):556–558
Turk O, Mavinic DS (1986) Preliminary assessment of a shortcut in nitrogen removal from wastewater. Can J Civ Eng 13(6):600–605
Turk O, Mavinic DS (1989) Maintaining nitrite build-up in a system acclimated to free ammonia. Water Res 23(11):1383–1388
Vadivelu V, Keller J, Yuan Z (2007) Free ammonia and free nitrous acid inhibition on the anabolic and catabolic processes of Nitrosomonas and Nitrobacter. Water Sci Technol 56(7):89–97
van Dongen UGJM, Jetten MS, Van Loosdrecht MCM (2001) The SHARON®-Anammox® process for treatment of ammonium rich wastewater. Water Sci Technol 44(1):153–160
Van Hulle SW, Vandeweyer HJ, Meesschaert BD, Vanrolleghem PA, Dejans P, Dumoulin A (2010) Engineering aspects and practical application of autotrophic nitrogen removal from nitrogen rich streams. Chem Eng J 162(1):1–20
Wu C, Chen Z, Liu X, Peng Y (2007) Nitrification–denitrification via nitrite in SBR using real-time control strategy when treating domestic wastewater. Biochem Eng J 36(2):87–92
Yang Z, Zhou S (2005) Investigation and analysis of hazardous constituents in landfill leachate from Datianshan landfill in Guangzhou. J Chem Ind Eng 56(11):2183–2188 (in Chinese)
Yang Q, Wang S, Yang A, Guo J, Bo F (2007) Advanced nitrogen removal using pilot-scale SBR with intelligent control system built on three layer network. Front Environ Sci Eng 1(1):33–38
Yoo H, Ahn KH, Lee HJ, Lee KH, Kwak YJ, Song KG (1999) Nitrogen removal from synthetic wastewater by simultaneous nitrification and denitrification (SND) via nitrite in an intermittently-aerated reactor. Water Res 33(1):145–154
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NSFC) (Grant No. 51178125, 51478127) and the S&T Planed Project of Guangdong Province (Grant No. 2012B030800005)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, C., Zhang, S., Zhang, L. et al. Effects of constant pH and unsteady pH at different free ammonia concentrations on shortcut nitrification for landfill leachate treatment. Appl Microbiol Biotechnol 99, 3707–3713 (2015). https://doi.org/10.1007/s00253-014-6340-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6340-0