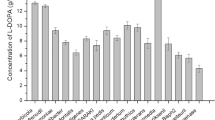
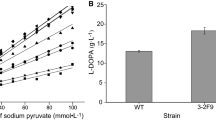
Abstract
l-DOPA (3,4-dihydroxyphenyl-l-alanine) has been widely used as a drug for Parkinson’s disease caused by deficiency of the neurotransmitter dopamine. Since Monsanto developed the commercial process for l-DOPA synthesis for the first time, most of currently supplied l-DOPA has been produced by the asymmetric method, especially asymmetric hydrogenation. However, the asymmetric synthesis shows critical limitations such as a poor conversion rate and a low enantioselectivity. Accordingly, alternative biotechnological approaches have been researched for overcoming the shortcomings: microbial fermentation using microorganisms with tyrosinase, tyrosine phenol-lyase, or p-hydroxyphenylacetate 3-hydroxylase activity and enzymatic conversion by immobilized tyrosinase. Actually, Ajinomoto Co. Ltd commercialized Erwinia herbicola fermentation to produce l-DOPA from catechol. In addition, the electroenzymatic conversion system was recently introduced as a newly emerging scheme. In this review, we aim to not only overview the biotechnological l-DOPA production methods, but also to briefly compare and analyze their advantages and drawbacks. Furthermore, we suggest the future potential of biotechnological l-DOPA production as an industrial process.






Similar content being viewed by others
References
Algieri C, Donato L, Bonacci P, Giorno L (2012) Tyrosinase immobilised on polyamide tubular membrane for the l-DOPA production: Total recycle and continuous reactor study. Biochem Eng J 66:14–19
Ali S, Haq IU, Qadeer MA, Rajoka MI (2005) Double mutant of Aspergillus oryzae for improved production of L-dopa (3,4-dihydroxyphenyl-L-alanine) from L-tyrosine. Biotechnol Appl Biochem 42:143–149
Ali S, Shultz J, Ikram-ul-Haq (2007) High performance microbiological transformation of L-tyrosine to L-dopa by Yarrowia lipolytica NRRL-143. BMC Biotech 7(1):50
Ates S, Cortenlioglu E, Bayraktar E, Mehmetoglu U (2007) Production of l-DOPA using Cu-alginate gel immobilized tyrosinase in a batch and packed bed reactor. Enzyme Microb Technol 40(4):683–687
Bielecki S, Bolek R (1996) Immobilization of recombinant E. coli cells with phenol-lyase activity. In: Wijffels RH, Buitelaar RM, Bucke C, Tramper J (eds) Progress in Biotechnology, vol 11. Elsevier, Amsterdam, pp 472–478
Fauziyah R, Gobikrishnan S, Indrawan N, Park S, Park J-H, Min K, Yoo YJ, Park D-H (2012) A study on electrochemical synthesis of L-DOPA using oxidoreductase enzymes: optimization of an electrochemical process. J Microbiol Biotechnol 22(10):1446–1451
Foor F, Morin N, Bostian KA (1993) Production of L-dihydroxyphenylalanine in Escherichia coli with the tyrosine phenol-lyase gene cloned from Erwinia herbicola. Appl Environ Microbiol 59(9):3070–3075
Iizumi K, Kotani T, Nishimoto Y, Tsuchida T (1991) Production of L-3,4-dihydroxyphenylalanine. Japan Patent JP5123177A
Ikram-ul H, Ali S, Qadeer MA (2002) Biosynthesis of l-DOPA by Aspergillus oryzae. Bioresour Technol 85(1):25–29
Katayama T, Suzuki H, Koyanagi T, Kumagai H (2000) Cloning and random mutagenesis of the Erwinia herbicola tyrR Gene for high-level expression of tyrosine phenol-lyase. Appl Environ Microbiol 66(11):4764–4771
Knowles WS (2004) Asymmetric Hydrogenations – The Monsanto L-Dopa Process. In: Blaser H-U, Schmidt E (eds) Asymmetric Catalysis on Industrial Scale. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, p 21–38
Koyanagi T, Katayama T, Suzuki H, Nakazawa H, Yokozeki K, Kumagai H (2005) Effective production of 3,4-dihydroxyphenyl-l-alanine (l-DOPA) with Erwinia herbicola cells carrying a mutant transcriptional regulator TyrR. J Biotechnol 115(3):303–306
Krishnaveni R, Rathod V, Thakur MS, Neelgund YF (2009) Transformation of L-Tyrosine to L-Dopa by a Novel Fungus, Acremonium rutilum, under submerged fermentation. Curr Microbiol 58(2):122–128
Lee J-Y, Xun L (1998) Novel biological process for L-DOPA Production from L-Tyrosine by P-Hydroxyphenylacetate 3-Hydroxylase. Biotechnol Lett 20(5):479–482
Lee S-G, Ro H-S, Hong S-P, Kim E-H, Sung M-H (1996) Production of L-DOPA by thermostable tyrosine phenol-lyase of a Themophilic Symbiobacterium species overexpressed in recombinant Escherichia coli. J Microbiol Biotechnol 6(2):98–102
Min K, Yoo YJ (2009) Amperometric detection of dopamine based on tyrosinase–SWNTs–Ppy composite electrode. Talanta 80(2):1007–1011
Min K, Park D-H, Yoo YJ (2010) Electroenzymatic synthesis of l-DOPA. J Biotechnol 146(1–2):40–44
Min K, Kim J, Park K, Yoo YJ (2012) Enzyme immobilization on carbon nanomaterials: Loading density investigation and zeta potential analysis. J Mol Catal B Enzym 83:87–93
Min K, Kathavarayan T, Park K, Yoo YJ (2013) Novel strategy for enhancing productivity in l-DOPA synthesis: the electroenzymatic approach using well-dispersed l-tyrosine. J Mol Catal B Enzym 90:87–90
Muñoz A, Hernández-Chávez G, Anda R, Martínez A, Bolívar F, Gosset G (2011) Metabolic engineering of Escherichia coli for improving l-3,4-dihydroxyphenylalanine (l-DOPA) synthesis from glucose. J Ind Microbiol Biotechnol 38(11):1845–1852
Nagatsu T, Sawada M (2009) L-dopa therapy for Parkinson's disease: Past, present, and future. Parkinsonism & Related Disorders 15. Suppl 1:S3–S8
Park H-S, Lee J-Y, Kim H-S (1998) Production of L-DOPA(3,4-dihydroxyphenyl-L-alanine) from benzene by using a hybrid pathway. Biotechnol Bioeng 58(2–3):339–343
Pialis P, Jimenez Hamann MC, Saville BA (1996) L-DOPA production from tyrosinase immobilized on nylon 6,6. Biotechnol Bioeng 51(2):141–147
Sayyed IA, Sudalai A (2004) Asymmetric synthesis of L-DOPA and (R)-selegiline via OsO4-catalyzed asymmetric dihydroxylation. Tetrahedron Asymmetry 15(19):3111–3116
Seetharam G, Saville BA (2002) l-DOPA production from tyrosinase immobilized on zeolite. Enzyme Microb Technol 31(6):747–753
Surwase S, Jadhav J (2011) Bioconversion of l-tyrosine to l-DOPA by a novel bacterium Bacillus sp. JPJ Amino Acids 41(2):495–506
Surwase S, Patil S, Apine O, Jadhav J (2012a) Efficient microbial conversion of l-Tyrosine to l-DOPA by Brevundimonas sp. SGJ. Appl Biochem Biotechnol 167(5):1015–1028
Surwase S, Patil S, Jadhav S, Jadhav J (2012b) Optimization of l-DOPA production by Brevundimonas sp. SGJ using response surface methodology. Microb Biotechnol 5(6):731–737
Valdes R, Puzer L, Gomes M, Marques C, Aranda D, Bastos M, Gernal A, Antunes O (2004) Production of L-DOPA under heterogeneous asymmetric catalysis. Catal Commun 5(10):631–634
Vilanova E, Manjon A, Iborra JL (1984) Tyrosine hydroxylase activity of immobilized tyrosinase on enzacryl-AA and CPG-AA supports: Stabilization and properties. Biotechnol Bioeng 26(11):1306–1312
Xu D-Y, Chen J-Y, Yang Z (2012) Use of cross-linked tyrosinase aggregates as catalyst for synthesis of l-DOPA. Biochem Eng J 63:88–94
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Min, K., Park, K., Park, DH. et al. Overview on the biotechnological production of l-DOPA. Appl Microbiol Biotechnol 99, 575–584 (2015). https://doi.org/10.1007/s00253-014-6215-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6215-4