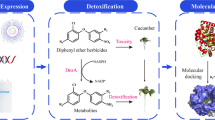
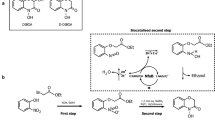
Abstract
Benzohydroxamic acids, such as 4-hydroxy-(2H)-1,4-benzoxazin-3(4H)-one (D-DIBOA), exhibit interesting herbicidal, fungicidal and bactericidal properties. Recently, the chemical synthesis of D-DIBOA has been simplified to only two steps. In a previous paper, we demonstrated that the second step could be replaced by a biotransformation using Escherichia coli to reduce the nitro group of the precursor, ethyl 2-(2′-nitrophenoxy)acetate and obtain D-DIBOA. The NfsA and NfsB nitroreductases and the NemA xenobiotic reductase of E. coli have the capacity to reduce one or two nitro groups from a wide variety of nitroaromatic compounds, which are similar to the precursor. By this reason, we hypothesised that these three enzymes could be involved in this biotransformation. We have analysed the biotransformation yield (BY) of mutant strains in which one, two or three of these genes were knocked out, showing that only in the double nfsA/nfsB and in the triple nfsA/nfsB/nemA mutants, the BY was 0%. These results suggested that NfsA and NfsB are responsible for the biotransformation in the tested conditions. To confirm this, the nfsA and nfsB open reading frames were cloned into the pBAD expression vector and transformed into the nfsA and nfsB single mutants, respectively. In both cases, the biotransformation capacity of the strains was recovered (6.09 ± 0.06% as in the wild-type strain) and incremented considerably when NfsA and NfsB were overexpressed (40.33% ± 9.42% and 59.68% ± 2.0% respectively).





Similar content being viewed by others
References
Blattner FR, Plunkett G, Bloch CA, Perna NT, Burland V, Riley M, ColladoVides J, Glasner JD, Rode CK, Mayhew GF, Gregor J, Davis NW, Kirkpatrick HA, Goeden MA, Rose DJ, Mau B, Shao Y (1997) The complete genome sequence of Escherichia coli K-12. Science 277:1453–1462
Bryant DW, Mccalla DR, Leeksma M, Laneuville P (1981) Type-I nitroreductases of Escherichia coli. Can J Microbiol 27:81–86
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA 97:6640–6645
Duke S (1986) Naturally occurring chemical compounds as herbicides. Rev Weed Sci 2:5–44
Esteve-Nuñez A, Caballero A, Ramos JL (2001) Biological degradation of 2,4,6-trinitrotoluene. Microbiol Mol Biol Rev 65:335–352
French CE, Nicklin S, Bruce NC (1998) Aerobic degradation of 2,4,6-trinitrotoluene by Enterobacter cloacae PB2 and by pentaerythritol tetranitrate reductase. Appl Environ Microbiol 64:2864–2868
González-Pérez MM, van Pieter D, Rolf MW, Ramos JL (2007) Escherichia coli has multiple enzymes that attack TNT and release nitrogen for growth. Environ Microbiol 9:1535–1540
Greenberg AE, Clesceri LS, Eaton AD (1992) Standard methods for the examination of water and wastewarer, 18th edn. APHA, Washington, 936
Guzman LM, Belin D, Carson MJ, Beckwith J (1995) Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol 177:4121–4130
Hamilton RH, Bandurski RS, Reusch WH (1962) Isolation and characterization of a cyclic hydroxamate from Zea mays. Cereal Chem 39:107–113
Honkanen E, Virtanen AI (1960) Precursors of benzoxazolinone in rye plants, II. Precursor I, the glucoside. Acta Chem Scand 14:504–507
Kobori T, Sasaki H, Lee WC, Zenno S, Saigo K, Murphy MEP, Tanokura M (2001) Structure and site-directed mutagenesis of a flavoprotein from Escherichia coli that reduces nitrocompounds—alteration of pyridine nucleotide binding by a single amino acid substitution. J Biol Chem 276:2816–2823
Kwak YH, Lee DS, Kim HB (2003) Vibrio harveyi nitroreductase is also a chromate reductase. Appl Environ Microbiol 69:4390–4395
Liochev SI, Hausladen A, Beyer WF, Fridovich I (1994) NADPH-ferredoxin oxidoreductase acts as a paraquat diaphorase and is a member of the SoxRS regulon. Proc Natl Acad Sci USA 91:1328–1331
Liochev SI, Hausladen A, Fridovich I (1999) Nitroreductase A is regulated as a member of the soxRS regulon of Escherichia coli. Proc Natl Acad Sci USA 96:3537–3539
Macías FA, Molinillo JMG, Galindo JCG, Varela RM, Simonet AM, Castellano D (2001) The use of allelopathic studies in the search for natural herbicides. J Crop Prod 4:237–255
Macías FA, Marín D, Oliveros-Bastidas A, Castellano D, Simonet AM, Molinillo JMG (2006a) Structure–activity relationship (SAR) studies of benzoxazinones, their degradation products, and analogues. Phytotoxicity on problematic weeds Avena fatua L. and Lolium rigidum Gaud. J Agric Food Chem 54:1040–1048
Macías FA, Marín D, Oliveros-Bastidas A, Chinchilla D, Simonet AM, Molinillo JMG (2006b) Isolation and synthesis of allelochemicals from Gramineae: benzoxazinones and related compounds. J Agric Food Chem 54:991–1000
Macías FA, Oliveros-Bastidas A, Marín D, Carrera C, Chinchilla N, Molinillo JMG (2008) Plant biocommunicators: their phytotoxicity, degradation studies and potential use as herbicide models. Phytochem Rev 7:179–194
Macías FA, Marín D, Oliveros-Bastidas A, Molinillo JMG (2009) Rediscovering the bioactivity and ecological role of 1,4-benzoxazinones. Nat Prod Rep 26:478–489
Michael NP, Brehm JK, Anlezark GM, Minton NP (1994) Physical characterization of the Escherichia coli-B gene encoding nitroreductase and its over-expression in Escherichia coli K12. FEMS Microbiol Lett 124:195–202
Rau J, Stolz A (2003) Oxygen-insensitive nitroreductases NfsA and NfsB of Escherichia coli function under anaerobic conditions as lawsone-dependent Azo reductases. Appl Environ Microbiol 69:3448–3455
Roldán MD, Pérez-Reinado E, Castillo F, Moreno-Vivián C (2008) Reduction of polynitroaromatic compounds: the bacterial nitroreductases. FEMS Microbiol Rev 32:474–500
Umezawa Y, Shimada T, Kori A, Yamada K, Ishihama A (2008) The uncharacterized transcription factor YdhM is the regulator of the nemA Gene, encoding N-ethylmaleimide reductase. J Bacteriol 190:5890–5897
Valle A, Cabrera G, Molinillo JMG, Gómez JM, Macías FA, Cantero D (2011) Biotransformation of ethyl 2-(2′-nitrophenoxy)acetate to benzohydroxamic acid (D-DIBOA) by Escherichia coli. Process Biochem 46:358–364
Whiteway J, Koziarz P, Veall J, Sandhu N, Kumar P, Hoecher B, Lambert IB (1998) Oxygen-insensitive nitroreductases: analysis of the roles of nfsA and nfsB in development of resistance to 5-nitrofuran derivatives in Escherichia coli. J Bacteriol 180:5529–5539
Williams RE, Bruce NC (2002) ‘New uses for an old enzyme’—the Old Yellow Enzyme family of flavoenzymes. Microbiol 148:1607–1614
Williams RE, Rathbone DA, Scrutton NS, Bruce NC (2004) Biotransformation of explosives by the old yellow enzyme family of flavoproteins. Appl Environ Microbiol 70:3566–3574
Yin H, Wood TK, Smets BF (2005) Reductive transformation of TNT by Escherichia coli: pathway description. Appl Microbiol Biotechnol 67:397–404
Zenno S, Koike H, Kumar AN, Jayaraman R, Tanokura M, Saigo K (1996a) Biochemical characterization of NfsA, the Escherichia coli major nitroreductase exhibiting a high amino acid sequence homology to Frp, a Vibrio harveyi flavin oxidoreductase. J Bacteriol 178:4508–4514
Zenno S, Koike H, Tanokura M, Saigo K (1996b) Gene cloning, purification, and characterization of NfsB, a minor oxygen-insensitive nitroreductase from Escherichia coli, similar in biochemical properties to FRaseI, the major flavin reductase in Vibrio fischeri. J Biochem 120:736–744
Zenno S, Kobori T, Tanokura M, Saigo K (1998) Conversion of NfsA, the major Escherichia coli nitroreductase, to a flavin reductase with an activity similar to that of Frp, a flavin reductase in Vibrio harveyi, by a single amino acid substitution. J Bacteriol 180:422–425
Acknowledgements
This work was supported by the Consejería de Innovación, Ciencia y Empresa de la Junta de Andalucía through the Project for Excellence P06-01399 (2006) and the PAIDI group CTS569; and the Co-operation Office of European Union in the LFA program called Bioprocess: Clean Technologies in the protection and sustainability of the environment with contract no.: AML/190901/06/18414/II-0548-FC-FA. The authors wish to thank PhD J.L. Ramos of the Consejo Superior de Investigaciones Científicas (CSIC) who provided the E. coli strain AB502NemA and Guillermo Gosset from the Instituto de Biotecnología from the Universidad Nacional Autónoma de Mexico (UNAM) for providing the detailed protocol for gene inactivation. Jorge Yañéz is acknowledged by primers synthesis.
Author information
Authors and Affiliations
Corresponding author
Additional information
A. Valle, S. Le Borgne and J. Bolívar have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Valle, A., Le Borgne, S., Bolívar, J. et al. Study of the role played by NfsA, NfsB nitroreductase and NemA flavin reductase from Escherichia coli in the conversion of ethyl 2-(2′-nitrophenoxy)acetate to 4-hydroxy-(2H)-1,4-benzoxazin-3(4H)-one (D-DIBOA), a benzohydroxamic acid with interesting biological properties. Appl Microbiol Biotechnol 94, 163–171 (2012). https://doi.org/10.1007/s00253-011-3787-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3787-0